sop for placebo batch
1.0 PURPOSE
To provide a procedure for numbering, manufacturing and packing of placebo batch and control to be followed for placebo batch.
2.0 SCOPE
2.1 Applicable to all placebo batches
3.1 References
In house
3.2 Attachments
3.2.1 Attachment- I : Batch Manufacturing Record of Placebo Batch for Analytical Purpose.
3.2.2 Attachment- II : Destruction Approval Form of placebo.
3.2.3 Attachment- III : Approval for Manufacturing of placebo Batch.
3.2.4 Attachment- IV : BMR/BPR Issuance/Closing Register of Placebo.
3.2.5 Attachment- V : Request of placebo for analytical purpose.
3.2.6 Attachment- VI : Batch number log for Placebo for analytical purpose.
4.0 DEFINITION
4.1 Definitions
4.1.1 Placebo Batch: A placebo batch of a product is processed as per regular formulation of that product except the active ingredient.
4.2 Abbreviations
4.2.1 NA : Not Applicable
4.2.2 SOP : Standard Operating Procedure
4.2.3 IT : Information Technology
4.2.4 EHS : Environment Health and Safety
4.2.5 EMRM : Excess Material Return memo
4.2.6 FAT : Factory Acceptance Test
4.2.7 ADL : Analytical Development Laboratory
4.2.8 BSR : Bonded Store Room
4.2.9 R&D : Research and Development
5.0 RESPONSIBILITY:
5.1 Production:
5.1.1 To raise “Approval of manufacturing of placebo batch”.
5.1.2 To compile the MBMR/MBPR.
5.1.3 To take the Material against consumption.
5.1.4 To check for reconciliation and conduct in-process checks at each stage of manufacturing and to record it in the BMR.
5.1.5 To raise the Analytical Request / Report.
5.1.6 To issue the batch for packing after release of batch.
5.1.7 To send the excess material through Excess Material Return Memo (EMRM).
5.2 Warehouse:
5.2.1 To dispense the raw materials and packing materials required for placebo batch.
5.2.2 To store the bulk finished placebo and pack finished placebo.
5.2.3 To receive the excess material through EMRM.
5.3 Packing:
5.3.1 To pack the bulk finished placebo as per requirement.
5.3.2 To check the overprinting proof and conduct in-process checks and record it in the BPR.
5.4 Quality Assurance:
5.4.1 To evaluate the “approval of manufacturing of placebo batch”
5.4.2 To review the MBMR and MBPR.
5.4.3 To take photocopy of BMR and BPR of placebo batch for analytical purpose.
5.4.4 To assign the batch number.
5.4.5 To ensure safe destruction of residue, recovery and manufacturing rejects of placebo batch.
5.4.6 To certify the EMRM.
5.5 Quality Control
5.5.1 To raise the request for manufacture of placebo batch for analytical purpose.
5.5.2 To sample /analyze bulk placebo as per Analytical Request / Report.
5.5.3 To label the reserve sample pack for placebo batch.
5.6 QA Head
5.6.1 To approve manufacturing of placebo batch.
5.6.2 To approve the MBMR and MBPR.
5.6.3 To authorize destruction of placebo batch.
5.7 EHS:
5.7.1 To ensure safe destruction of residue, recovery and manufacturing rejects of placebo batch and the placebo.
5.7.2 To authorize the destruction of the placebo batch.
5.8 Plant Head
5.8.1 To acknowledge the approval of manufacturing of placebo batch.
5.8.2 To acknowledge the destruction of the placebo.5.9 Research and Development (R&D)
5.9.1 To approve the MBMR and MBPR whenever placebo batch is manufactured based on R and D requirement.
6.0 Distribution:
I. :Quality Assurance
II. : Quality Control
III. : Production
IV. :Warehouse
V. :EHS
7.0 PROCEDURE:
7.1 Placebo batch shall be manufactured based on the requirement from:
7.1.1 Medical service department for clinical trials.
7.1.2 Quality control department.
7.1.3 Production /packaging department for new equipment /Machine change part trial/ new pack size.
7.1.4 R&D/ Formulation development/Improvements/ADL.
7.1.5 For qualification of equipment’s.
7.1.6 Marketing department for demonstration purpose.
7.1.7 As per customer requirement.
7.1.8 For transportation trial.
Note: This is not a comprehensive list by itself. There may be other requirement which need to be approved by QA Head.
7.2 Approval/ Authorization of Placebo batch:
7.2.1 As per the requirement an “Approval for manufacturing of placebo Batch” shall be raised by the production Department. Refer Attachment – III.
7.2.2 Approval for manufacturing of placebo batch shall be evaluated by the Quality Assurance Department, approved by QA Head.
7.2.3 After receipt of the approval, production shall prepare MBMR and release for approval to QA. After checking the draft BMR QA shall take print out.
7.3 Batch Numbering system for Placebo batch:
7.3.1 Batch numbering system for placebo batch shall be as follow:
The batch number shall consist of three alphabets prefix followed by four digit numbers.
7.4 Batch numbering system for Placebo batch manufactured for Analytical Purpose:
7.4.1 Batch numbering system shall be as follow:
The batch number shall consist of three alphabets prefix followed by four digit numbers.
7.5 Batch record shall be requested by the user department as per Document Request Form.
Separate batch number log shall be maintained for placebo batches. (Refer Attachment-IV)
7.6 Manufacturing of placebo batch:
7.6.1 The placebo Master batch manufacturing Record shall be compiled by Production department, approved by Quality Assurance and authorized by QA Head.
7.6.2 The placebo Batch shall be manufactured as per the instructions given in the BMR. Raw materials required for placebo batch shall be dispensed as per work order. Each stage of manufacturing shall be checked for reconciliation and in process checks and recorded in the BMR by production officer.
7.6.3 The stage up-to which the placebo to be manufactured shall be decided as per the requirement.
7.6.4 After completion of batch manufacturing, the bulk finished placebo shall be packed as per the requirement. The bulk shall be labeled as “Bulk Finished placebo” as per SOP titled as “Status Labeling” and stored under lock and key.
7.6.5 The residual recovery and manufacturing rejects of the batch shall be destroyed by production officer under supervision of quality
assurance and EHS officer.
7.6.6 Production officer shall hand over the batch for intended purpose to concerned department such as ADL, Quality control, R&D and
packing.
7.6.7 The placebo batch after completion of intended purpose shall be reconciled and destroyed by taking approval as per Attachment- II.
7.6.8 In case of placebo manufactured for FAT /Trial at party level or as per any other requirement, the same shall be sent out of the
premises by routine through warehouse.
7.7 Packing of placebo batch:
7.7.1 After completion of batch manufacturing, Analytical Request/Report shall be raised by production department and sample shall be collected by QA with details of tests required to be performed and sample shall be sent to QC department for analysis of bulk placebo.
7.7.2 After the placebo batch is released by Quality Assurance, production officer shall issue the batch to Packaging Department.
7.7.3 A placebo Master Batch Packing Record shall be compiled by Production Department,
Approved by Quality Assurance and authorized by QA Head and batch packing can be executed based on approved master.
7.7.4 The line clearance of the area and the machine where the placebo batch is packed shall be as per the respective SOP.
7.7.5 All overprinted or plain packaging material specimens of the batch shall be as per respective SOP.
7.7.6 All in process checks during packing activity shall be recorded in the batch packing record by the packing officer.
7.7.7 After the batch packing is completed, the excess material if any shall be sent to Warehouse through EMRM
as per the SOP or destroyed after the certification of QA.
7.7.8 On line packing rejects, machine rejects, residual recovery of the batch shall be destroyed as per the respective SOP.
7.7.9 After the placebo batch is released by QA, packing department shall deliver the batch to BSR.
7.7.10 No samples of placebo batch shall be made available at manufacturing locations except reserve
samples of placebo or placebo required for routine analysis and stability analysis in QC department of that location.
Reserve samples pack of the placebo batch shall have additional label as “Placebo Batch” The label shall contain the following details:
Placebo batch for: Batch Number: Mfg. Date:
7.8 Procedure for manufacturing of Placebo batch for analytical Purpose:
7.8.1 A placebo batch can be manufactured based on the requirements of Quality control.
7.8.2 Quality control shall raise a request for placebo batch.
7.8.3 On receipt of request from Quality control (Attachment-V), production department
shall raise approval for manufacturing of placebo batch as per Attachment –III.
7.8.4 Approval for manufacturing of placebo batch shall be evaluated by QA, approved by Head QA.
7.8.5 After approval production shall send the request and QA shall assign a batch number on
the approval page as per defined procedure at 7.4.
7.8.6 After receipt of approval QA shall take a photocopy of the manufacturing record
of placebo for analytical purpose. (Refer Attachment- I).
7.8.7 Product name, batch number, Batch size and manufacturing date shall be written in batch manufacturing record.
7.8.8 Production officer shall write the item code, name of the ingredients required along with the
Pharmacopoeial grade, quantity, and unit of measurement in the manufacturing record of the placebo for analytical purpose.
7.8.9 The item code of the materials used for the placebo preparation shall be the same as per the respective product MBMR and quantity shall be proportionate to the respective product MBMR.
7.8.10 Above details shall be verified by QA before issue.
7.8.11 After authorization of the BMR, the dispensing of excipients shall be done as per the dispensing SOP.
7.8.12 In case of solid dosage forms, mix the ingredients in the same sequence as given in
the respective Product MBMR .Use a suitable SS container or poly bag for the same. In case of the product
formulation follow the sequence of manufacturing as given in the respective MBMR.
7.8.13 Label the placebo with “Placebo for Analytical Purpose” label as per SOP titled as “Status Labeling”.
7.8.14 Send the labeled placebo to the respective section of Quality control.
7.8.15 Quality control shall maintain the usage log of placebo (Attachment-VI) and the remaining (excess) quantity,
if any, of placebo at unit level. Placebo shall be destroyed by quality control.
Note: A validity of placebo batch shall be same as respective product shelf life. Placebo for stability
shall be retained up to last interval of stability.
Attachment- I
BATCH MANUFACTURING RECORD OF PLACEBO BATCH FOR ANALYTICAL PURPOSE
Product Name: Placebo For Batch No.: Batch Size in g/Kg/ L: Mfg. Date:
Issued By: Approved By:
Date: Date:
Manufacturing Issue material Order Stage A: Dispensing of Raw materials
Caution:
1. Use snood and gloves while handling the materials.
2. Cleaned and dried containers, accessories shall be used for dispensing.
3. Dispensing shall be done under RLAF.
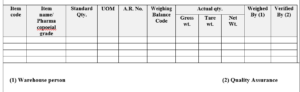
Stage B: Manufacturing Details:
Caution: 1. Use snood & gloves while handling the material Cleanliness of the container verified By /Date:
Fresh poly-bag shall be used (if poly-bag is used), verified by/date:
Procedure:
1. Transfer dispensed material to manufacturing area.
2. Add ingredients in the same sequence as per product MBMR. Mix all the ingredients using suitable equipment or poly bag and visually ensure uniform mixing.
3. Transfer the placebo into clean double poly-bags or in a SS container or any other suitable container and seal the container properly.
4. Label the container/poly-bag of placebo. (As per SOP titled as “Status Labeling”).
5. Weigh the placebo and record in the reconciliation form.
Attachment- II
DESTRUCTION APPROVAL FORM OF PLACEBO
We would like to dispose the quantity as per SOP No.: . Placebo for product:
Batch No. : Manufacturing Date: Quantity:
Cost:
Reason:
Kindly authorize us to do the same Proposed By: Date:
EHS comments:
Attachment- III
APPROVAL FOR MANUFACTURING OF PLACEBO BATCH
Placebo for Product (Name):
I) Placebo Batch Required For:
II) Methodology of Implementation:
Proposed By: Date: Evaluated By: Date: (Production) (QA)
Approved By: Date: Authorized By: Date: (QA Head) (Plant Head)
Batch Number: Date: Issued By (QA):
Attachment- IV
BMR / BPR ISSUANCE/CLOSING REGISTER OF PLACEBO

Attachment- V
REQUEST OF PLACEBO FOR ANALYTICAL PURPOSE
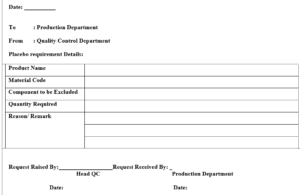
..
Attachment- VI
BATCH NUMBER LOG FOR PLACEBO FOR ANALYTICAL PURPOSE

Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt
sop for Cleaning and operation of spray gun and assemble
sop for Fogging in Aseptic and Non Aseptic Area