sop for Fogging in Aseptic and Non Aseptic Area
1.0 OBJECTIVE:
1.1 To lay down a procedure for Fogging in Aseptic and Non Aseptic Area.
2.0 SCOPE:
2.1 This SOP is applicable for Fogging in Aseptic and Non Aseptic Area in Injection Parenteral
3.0 RESPONSIBILITY:
3.1 Officer / Executive Production
4.0 ACCOUNTABILITY:
4.1 Head Production
5.0 PROCEDURE:
5.1 PROCEDURE:
5.1.1 Operator/Staff/Helper shall be wear goggles during fogging and leave the area after start the fogger.
5.1.2 Area AHU should be OFF before 5 min of fogging and should be start after 30 min of fogging.
5.2 Check & ensure that the area intended for fogging is completely closed.
5.3 Silvicide shall be used as Fogging Agent.
5.4 20% Solution of Silvicide in WFI shall be used as Fogging Solution. (For 1000 cu. ft. Area to be fogged
with Fogger – 200 ml of Silvicide in 800 ml WFI to be used).
5.5 Fogging with 20% Silvicide Solution shall be performed in the particular Area.
5.6 After Fogging, leave the Area in Static Condition up to one hour for complete sanitization.
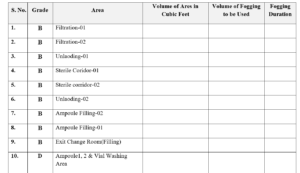
5.7 Area should be fogged with defined time which is mention in Annexure-III, Titled “Fogging Solution Usage Record”.
5.8 Clean the Area and Equipment before starting of any activity with scheduled Disinfectant Solution for the day.
5.9 Record the Fogging details in Annexure-I, Titled “Fogging Record of Aseptic Area and Annexure-II, Titled “Fogging Record of Manufacturing/Washing & Sterilization/Unit preaparation
5.10 Frequency:
5.10.1 Daily and after Operation
Fogging frequency may be increased on the basis of Environmental Monitoring Report of the said Area.
Shut Down
Microbial Count (Beyond Limit)
6.0 ABBREVIATIONS:
| Sr. No. | Abbreviation used | Full form of Abbreviation used |
| 1. | SOP | Standard Operation Procedure |
| 2. | Ltd. | Limited |
| 3. | WFI | Water for Injection |
| 4. | Sr. No. | Serial Number |
| 5. | QA | Quality Assurance |
| 6. | QC | Quality Control |
| 7. | Cu. Ft. | Cubic feet |
7.0 ATTACHMENTS (ANNEXES) :
Annexes-I : Fogging Record
Annexes-II : Fogging Record of Manufacturing/Washing and Sterilization/Unit preaparation.
Annexes-III : Fogging Solution Usage Record.
8.0 REFERENCES:
| S. No. | Reference Title |
| 1. | In-House |
Annexes-I
FOGGING RECORD OF ASEPTIC AREA
Department: Production –Fogging Agent:
Date:
Frequency: Daily and after operation
|
Area |
Time |
Done By |
Checked By | |
| From | To | |||
| Ampoule Filling-III
|
||||
| Ampoule Filling-II
|
||||
| Ampoule Filling –I
|
||||
| Buffer Zone | ||||
| Unloading room-II | ||||
| Unloading room-I | ||||
| Filtration-I | ||||
| Filtration-II | ||||
| Sterile corridor | ||||
| Air Lock -IV | ||||
| Air Lock-III | ||||
| Air Lock -II | ||||
| Air Lock –V | ||||
| Air Lock -I | ||||
Annexes-II
FOGGING RECORD OF MANUFACTURING/AMPOULE WASHING/UNIT PREPARATION
Department: Production Area:
Month: Fogging Agent:
Year: – Frequency: Weekly ±1Day
| Date | Area | Fogging Time | Done By
Sign & Date |
Checked By
Sign & Date |
Remarks | |
| From | To | |||||
Annexes-III : Fogging Solution Usage Record
Online Rejection in parenteral
Receipt of Batch from Production to Packing Department
sop for for Spillage Handling in parenteral area
sop for calibration of vessels with dipstick
sop for Cleaning of Bins and Containers
cip of mixing vessel and holding vessel
sop for Cleaning of Ampoule Filling and Sealing Machine
sop for Fogging in Sterile and Non Sterile Area
sop for for Filtration of Bulk Solution
sop for fumigation in production area
sop for post cleaning after media fill
sop for cip of mixing vessel mixing mobile vessel and holding vessel
sop for De-Bagging of Three Piece Vial Dropper Caps
sop for calibration and verification of check weigher
sop for Batch number and Manufacturing and Expiry Date Coding System
standard operating procedure machine history file
sop for operation and cleaning of Hand coder
sop for Cleaning and Handling and Silicone Tubes
sop on operation and cleaning of coating pan
sop for Operation of cleaning of pipe lines
sop for operation of capsule loading machine semi automatic
sop for Machine operation capsule inspection and polishing machine
Sop batch demarcation and batch coding
sop for monitoring of reprocessing of products
sop for in-process control on liquids orals
sop for in process controls on tablets capsules packaging line
sop for Issuance retrieval and destruction of BMR and analytical records
sop for in process controls during granulation compression coating inspection
sop for Cleaning of Blister packing machine
sop for for charge hand over between the shifts
Performance requalification report of visual inspectors
sop for Cleaning and operation of ROPP caps inspection table
sop for usage and destruction of filter pad and cartridge filter
sop for cleaning and storage of transfer pipe
sop for Cleaning and operation of labeling machine
Cleaning and operation of the mono block filling and sealing machine
sop for Cleaning and operation of empty bottle inspection table
sop for Cleaning and operation of filter press
sop for cleaning and operation of liquid transfer pump and line
sop for cleaning and operation of storage vessels
sop for cleaning and operation of sugar syrup manufacturing vessel
sop for cleaning issuance and retrieval of accessories and change parts
sop for Cleaning and operation of visual inspection conveyor belt
sop for Cleaning and operation of spray gun and assemble