Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
1.0 OBJECTIVE
1.1 To lay down a procedure for analysis of Ferrous Gluconate, Calcium Gluconate, Vitamin D3 Cyanocobalamin (Vit.B12) & Folic Acid Suspension.
2.0 SCOPE
2.1 This procedure is applicable to the analysis of Ferrous Gluconate, Calcium Gluconate, Vitamin D3 Cyanocobalamin (Vit.B12) & Folic Acid Suspension in Quality control
3.0 RESPONSIBILITY
3.1 Q.C- Chemist
4.0 ACCOUNTABILITY
4.1 Manager-Quality Assurance
5.0 PROCEDURE
5.1 Description: Pour 50 ml finish sample in beaker and observed visually.
5.2 pH: Taken 50 ml sample in beaker rinse the pH electrode first with purified water followed by sample dip the electrode in sample and observed the pH.
5.3 Volume variation: Measured the volume by measuring cylinder and determine the volume variation.
5.4 Identification:
5.4.1 In the assay, the principle peak in the chromatogram obtain with the test solution correspond to the peak in the chromatogram obtained with the reference solution. and by chemical method.
5.5 ASSAY:
Each 5ml contains:
Ferrous Gluconate I.P.
eq.to Elemental Iron. 50.0mg.
Calcium Gluconate I.P. 125.0mg.
Vitamin D3 I.P. 200IU
Folic Acid I.P. 1.0mg.
Vitamin B12 I.P. 5.0mcg.
Estimation of Ferrous Gluconate, Calcium Gluconate, Vitamin D3, Folic Acid &Vitamin B12 Suspension.
Method of Ferrous Gluconate Eq.to Elemental Iron.
Method of Iron:
Weigh accurately equivalent to 50 mg of Iron and 5 ml water. add 3ml sulphuric acid. heat and cool. Add potassium permanganate solution till. Changes the colour pale yellow or disappear colour add 2 gm potassium iodide. After that 25 ml Hcl stay on 5 to 10 minute. Add starch solution as indicator. Titrate with 0.1 M sodium Thiosulphate.
Factor-5.85 mg
V= Volume (in ml) of Sodium Thiosulphate. Solution consumed in titration.
N = Actual Normality of Sodium Thiosulphate Solution.
AW= Average weight
Wt. = Weight of Sample.
Calculation:

Method of Calcium Gluconate:
Taken equivalent to 500 mg of Calcium Gluconate add 50 ml water, cool, add 5.0 ml of Magnesium Sulphate and 10 ml of strong ammonia solution and titrate with 0.05 M Disodium edetate. Using mordent black II mixture as indicator. Factor-0.002004 g. of calcium.
V= Volume (in ml) of 0.05M Disodium edetate. Solution consumed in titration.
N = Actual Normality of 0.05M Disodium edetate Solution.
AW= Average weight
Wt. = Weight of Sample.
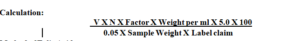
Method of Folic Acid.
Buffer: 680 mg potassium dihydrogen orthophosphate dissolve in 100 ml water. Adjust to ph-6.0 with
1N sodium hydroxide.
Mobile phase: Buffer: methanol
90 : 10
Standard preparation: weigh accurately 50 mg of folic acid in 100 ml volumetric with 0.1N Sodium hydroxide. Further dilution 1.0ml to 50 ml with mobile phase.
Sample preparation: weight accurately equivalent to 1.0mg of sample weight add 10 ml of 0.1 N Sodium hydroxide shake for 5 minute. And diluted to 100 ml volumetric flask
With mobile phase.
Chromatographic condition:
Wavelength -280 nm
Column -C18 (250 x4.6) mm
Flow -1.2ml per minute.
Injection volume -10 micro liter.
Retention time about 15 mint.
Temperature – Ambient

Method of Cholecalciferol (vitamin D3)
BY HPLC
Diluted solvent: Methanol
Mobile phase: METHANOL: ACETONITRILE
90: 10
Standard preparation: Weight accurately about 25 mg std wt. of vitamin D3 in 100 ml volumetric flask.
Further dilution 2.0 ml to 100 ml volumetric flask with methanol.
Sample preparation: Weight accurately Equivalent to 0.5 mg of vitamin D3 and diluted TO 100 ml with methanol.
Chromatographic condition:
Wavelength -290nm
Flow rate – 1.0 ml /mint.
Column -c18 (150 x4.6) 5µm.
Temp – 350c
Retention time – about 5.70 mint
Injection volume – 100µl.

Estimation of Cyanocobalamin (Vitamin B12)
CULTURE: E. coli mutant NCIM 2567 as a test organism. The culture is maintained by sub-culturing. It on a slant of E. coli maintenance Agar medium, once a week.
REAGENTS:
1. B12 Culture agar (E. coli maintenance medium)-Hi-media M-185
2. B12 Assay agar using (E. coli maintenance medium)-Hi-media M-110.
PREPARATION OF STOCK SOLUTION:
Weigh accurately about 100mg Vitamin B12 reference standard and dissolve in sufficient water to produce 100ml.
This solution may be preserved in a refrigerator for one year.
PREPARATION OF STOCK STANDARD SOLUTION:
Dilute 2 ml of the stock solution to 100ml with water. I.e., 20mcg/ml dilution. This stock solution can be preserved for one month in a refrigerator.
PREPARATION OF WORKING STANDARD:
This stock standard is allowed to come to room temperature on the day of the assay.
0.5ml of stock standard is further diluted to 100ml with water to get 100 Nanograms vitamin b12.
One ml of the 100 Nanograms solution is diluted to 4ml to get 25 Nanograms per ml. use 25 Nanograms per ml and 100 Nanograms per ml in assay.
PREPARATION OF SAMPLE DILUTION:
Prepare the Sample suitably diluted with water to produce 25 Nanograms per ml and 100 Nanograms per ml of vitamin b12., filter the solution if necessary.
PREPARATION OF CULTURE SUSPENSION:
To 18-24 hours’ culture of E. coli mutant, add 10ml of sterile water or normal saline to make suspension. B12 assay agar is weighed and autoclaved for 15min at 15 pounds, cool to 45-degree cent. Add 1.5 ml to 2ml of culture suspension to 100ml of assay agar and mix well. Add 20 to 25ml of this agar is poured in to sterile Petri dishes of size 100mm in diameter. Allow to solidify. Make four cups is agar at proper distance to avoid overlapping of zones of inhibition. Sterilized borer of 5mm to 10mm in diameter is used to bore cups. Add 0.05 or 0.1 ml of standard solution of 100 Nanograms per ml (SH) and 25 Nanograms per ml (SL) dilutions to each agar cup.
Similarity add 0.05 or 0.1ml of test solution of 100 Nanograms per ml (TH) and 25 Nanograms per ml (TL) dilution to each agar cup labeled as test high and test low.
Incubation:
Cover the Petri dishes, invert and incubate them at 320C to 350C for 18 to 24 hours.
Calculation:
Measure the zones and calculate vitamin b12 content accordingly to the following formula:
%Assay = Antilog (2± a Log I)
Where: a= ( TH + TL) – ( SH + SL)
(TH- TL) + (SH – SL)
I = Ratio of dilution i.e. 10/25
TH= Zone of test sample with sample 100 Nanograms per ml
Tl = Zone of test sample with sample 25 Nanograms per ml
SH= Zone of standard with sample 100 Nanograms per ml
SL= Zone of standard with sample 25 Nanograms per ml
6.0 Abbreviation
| Sr. No. | Abbreviation used | Full form of abbreviation used |
| 1.0 | STP | Standard Testing Procedure |
| 2.0 | QA | Quality assurance |
| 3.0 | STD | Standard |
| 4.0 | SPL | Sample |
| 5.0 | NM | Nano Meter |
7.0 (ANNEXURE)
NA
8.0 REFERENCE
| Sr. No. | Reference Title |
| 01 | In House |
Bacterial endotoxin test for dexamethasone injection
Analysis of vitamin B1 B6 B2 Nicotinamide and sodium pentothenate Injection
Analysis for Nandrolone Decanoate injection
Analysis of Dicyclomine and Diclofenac sodium Injection
standard testing procedure of Fexofenadine and Phenylephrine suspension
standard testing procedure of Piroxicam Injection
STP of Fungal Diastase and Papain capsules
standard testing procedure PVC
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
Analysis method of aceclofenac
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
Analysis method of Suspension of Tribasic Calcium phosphate with vitamin D3 and Vitamin B12
Analysis method of Calcitriol with calcium citrate Suspension
Analysis method of Oxyclozanide and Fenbendazole Suspension
Analysis method of Oxyclozanide and Levamisole Suspension
Analysis method of Triclabendazole and Ivermectin Suspension
Analysis method of Itraconazole Solution
Analysis method of Levocetirizine Dihydrochloride syrup
Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
Analysis method of Ferrous Ascorbate Cyanocobalamin and Folic Acid Suspension
Analysis method of Ambroxol Hydrochloride Drops
Analysis method of Ferrous Ascorbate with Folic Acid suspension
Analysis method of Piracetam Syrup
Analysis method of Rafoxanide and Levamisole suspension
Analysis method of Zinc gluconate Syrup
Analysis method of Magaldrate Simethicone and Oxetacaine suspension
Analysis method of mefenamic acid and paracetamol suspension
Analysis method of Cholecalciferol Drops
Analysis method of Racecadotril suspension
Analysis method of Deflazacort Suspension
Analysis method of Montelukast sodium and levocetirizine Dihydrochloride Syrup
Analysis method of Iron and Folic Acid Syrup
Analysis method of Cyproheptadine Hydrochloride and Tricholine Citrate Syrup
Analysis method of Levofloxacin Hemihydrate Ornidazole and Vitamin E Solution
Analysis method of Albendazole and ivermectin in oral liquid