Analysis method of Piroxicam and paracetamol Injection
1.0 OBJECTIVE
1.1 To lay down a procedure for Analysis of Piroxicam and paracetamol Injection
2.0 SCOPE
2.1 This procedure is applicable to the analysis of Analysis of Piroxicam & paracetamol Injection in QC lab
3.0 RESPONSIBILITY
3.1 Q.C- Chemist
4.0 ACCOUNTABILITY
4.1 Manager-Quality Assurance
5.0 PROCEDURE
5.1 Identification:
5.2 Description: Pour 50ml finish sample in Beaker and observed Visually.
5.3 pH: Taken 50ml sample in beaker rinse the pH electrode first with purified water followed by sample dip the electrode in sample and observe the pH.
5.4 Volume variation: Measured the volume by syringe and determine the volume variation.
5.5 Sterility:
MEMBRANE FILTRATION METHOD
5.5.1 Wipe the sample article individually with 70% IPA solution and keep in a clean S.S trays marked with Product Name, Batch No. and Lot No, and then transfer the samples to the sterility room through clean pass box for performing sterility.
5.5.2 Prepare the media tubes (FTM and SCDM) as per the SOP for preparation of culture media
Dispense 100 ml quantity for membrane filtration and for Direct Inoculation Method. Sterilize both
the media at 1210C and 15 psi pressure for 20 minutes as per SOP for Media Sterilization by Autoclaving.
5.5.3 After autoclave Label the tubes with Name of Media, Media Batch No. and pre-incubate the media tubes at appropriate temperature i.e. SCDM tubes at 20 to 250C whereas FTM tubes at 30 to 350C for 24 – 48 hrs. before subjecting them for sterility operations.
5.5.4 Autoclave Dress, Scissors, forceps and Filtration unit in a S.S Container at 1210C temperature and 15psi pressure for 30 minutes.
5.5.5 After Sterilization cool the contents and aseptically transfer in a S.S. container to cleaned Pass box.
5.5.6 Transfer the pre incubated sterile media tubes, SCDA plates, sterile swabs, sterilized Filtration unit, and 0.1% w/v peptone water to the sterility test room through pass box.
5.5.7 Enter in sterility room as per the Entry / Exit procedure for Sterility Room.
5.5.8 Start the LAF as per SOP.
5.5.9 Wipe out all samples to be tested for sterility with 70% filtered IPA solution.
5.5.10 Before starting sterility test, expose the SCDA plates as specified locations throughout the testing.
5.5.11 Connect the Glass filtration cup holder with the Filtration flask properly with pipe under laminar airflow unit.
5.5.12 Check the Manometer reading of working LAF and check the temperature as well as humidity of the sterility room.
5.5.13 Switch ON the vacuum pump with the help of switch, present on the wall. Now place 0.45 sterile membrane filters between filtration cup and receptacle with the help of sterilized forceps and clamped it properly.
5.5.14 Wet the membrane filter by adding approx. 15 ml of sterilized Fluid A (0.1% peptone water) to filter holder, and filter the fluid by employing vacuum.
5.5.15 Cut the tip of bottle/vial or ampoule with sterile SS blade in front of the gas burner and immediately transfer the contents of sample to membrane filter. For dry powder or lyophilized container, add the sterile water 0.1% peptone dissolve and then collect it in sterile flask and immediately transfer the contents of sample to membrane filter.
5.5.16 Immediately filter the solution with the aid of vacuum and wash the membrane three times with 100 ml of sterilized fluid A (0.1% peptone water).
5.5.17 After complete filtration, stop the vacuum pump.
5.5.18 Lift the membrane carefully with the help of sterile forceps, aseptically cut the membrane filter into two halves with sterile SS scissor and transfer one half to FTM and one half to SCDM tubes by unplugging in front of gas burner only.
5.5.19 Label both the tubes with Product name, B. No, Report No.., date of testing, Completion date & Tested by.
5.5.20 Simultaneously prepare a negative control by filtering 100 ml of 0.1% peptone water
5.5.21 Instead of product sample, cut the membrane into two halves with sterile SS scissor and transfer one half to FTM and one half to SCDM and label both the tubes as Negative control.
5.5.22 For media negative control, keep one – one tube of each autoclaved lot of FTM & SCDM un-inoculated.
5.5.23 After completion of work transfer all inoculated media through hatch box and then transfers all the equipment and exposed plates to microbiology analysis section.
5.5.24 Incubate the FTM tubes at 300C – 350C and SCDM tubes at 200C – 250C. Incubation period for terminally sterilized products are 7 days and 14 days for aseptically filled products as per IP. If the Product is as per USP, BP, incubation period is 14 days for both terminally sterilized as well as for aseptically filled products.
5.5.25 Start the LAF of Biosafety cabinet as per SOP and prepare three positive Control tubes by inoculating aseptically not more than 100 cfu in FTM tubes with S. aureus, P. aeruginosa and Clostridium sporogenes. Similarly prepare three SCDM positive control by inoculating not more than 100 cfu separately with C. albicans, A. Niger, and Bacillus subtilis. Incubate FTM positive control tubes at 30 – 35ºC & SCDM positive control tubes at 20 – 25ºC. For bacterial positive controls, incubation period is not more than 3 days & for fungal positive control, incubation period is not more than 5 days.
5.5.26 Bacterial endotoxin test
PROCEDURE FOR TESTING:

5.6.3 Dilute the sample MVD/2 times with LAL water when performing the test at MVD. If performing the test at MVD/2, dilute the sample MVD/4time with LAL water.
5.7 Preparation of E-Coli Control Standard Endotoxin (CSE):
5.7.1 Reconstitute the CSE with appropriate volume (as stated on certificate) of LAL reagent water vortex continuous 30 minutes at the interval of 10minutes (When new bottles reconstitute) & vortex vigorously for five minutes before further dilution
5.8 Preparation of CSE dilution series:
5.8.1 Confirm the labeled sensitivity using at least one vial of each batch/lot of lysate. Prepare a series
of two-fold dilution of the CSE to give concentrations of 2, , 0.5 and 0.25, where is the labeled sensitivity of the lysate in EU per ml.
5.8.2 Perform the test as given as under Procedure on these four standard concentrations in duplicate or greater in include negative control consisting of water BET.
5.8.3 Calculate the average of the logarithms of the lowest concentration of endotoxin in each series of dilutions for which a positive result is found. The antilogarithm of this average gives the estimated lysate sensitivity, which must be greater than or equal to 0.5 and less than or equal to 2.0.
confirm the labeled sensitivity of each new batch of lysate prior to use in the test. Test CSE results are only valid when the positive water and specimen controls are positive at the 2 & endotoxin concentration.
5.9 Preparation of LAL Reagents:
5.9.1 Test LAL reagent for its sensitivity at the time of receiving. For reconstitution, Collect LAL powder into the bottom of the vial by tapping on a firm surface, unseal and release the vacuum by slowly lifting the stopper, avoiding touch contamination. The small amount of LAL on the stopper is insignificant.
5.9.2 Rehydrate the reagent with appropriate volume of (as stated on label and certificate) LAL reagent water by pipetting directly into the vial immediately before use. Cover the vial with an endotoxin free surface or
the inner side of perafilm when not in immediate use. Gently swirl until LAL dissolves into a colorless solution.
5.10 Test procedure for gel clot:
5.10.1 Each assay should include dilutions of a sample or product, a negative water control, a positive product control, and either a 2 water control or a series of two-fold dilutions from an endotoxin standard which bracket the labeled LAL sensitivity.

5.11 Interpretation of results:
5.11.1 Each tube in the gel-clot method is interpreted as either positive or negative. A positive test is defined as formation of a firm gel capable of maintaining its integrity when the test tube is inverted 180°C. A negative test is characterized by the absence of gel or by the formation of a viscous mass, which does not hold when the tube is inverted. Calculate sample endotoxin concentration by the following formula.

5.12 ASSAY:
Each 1.0 ml contains:
Piroxicam IP 20 mg.
Paracetamol IP 100mg.
Estimation of Piroxicam & Paracetamol:
Method of Piroxicam & Paracetamol:
BY HPLC
Buffer: 2.5 ml Orthophoshoric Acid produced to 1000 ml water.
Mobile phase: Buffer : Acetonitrile
30 : 70
Standard preparation: Weight 100 mg of Paracetamol and 20mg. Piroxicam diluted to 100 ml volumetric flask with Mobile phase.
Sample preparation: Taken equivalent to 100 mg of Paracetamol and 20mg. Piroxicam diluted to 100 ml volumetric flask volumetric flask with Mobile phase.
Chromatographic condition:
Wavelength -272nm
Flow rate – 1.0ml /mint.
column -C18 (250mm x4.6) 5µm.
Temperature -Ambient.
Injection Volume -20µl.
Retention time of paracetamol about 2.15 mint.
Retention time of piroxicam about 3.0 mint
Calculation:
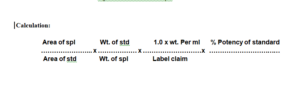
6.0 ABBREVIATIONS
| Sr. No. | Abbreviation used. | Full form of abbreviation used. |
| 1.0 | STP | Standard Testing Procedure. |
| 2.0 | QA | Quality assurance. |
| 3.0 | STD | Standard. |
| 4.0 | SPL | Sample |
| 5.0 | I.P. | Indian Pharmacopeia |
| 6.0 | NM | Nano meter |
7.0 (ANNEXURE)
NA
8.0 REFERENCE
| Sr. No. | Reference Title |
| 01 | In House |
Bacterial endotoxin test for dexamethasone injection
Analysis of vitamin B1 B6 B2 Nicotinamide and sodium pentothenate Injection
Analysis for Nandrolone Decanoate injection
Analysis of Dicyclomine and Diclofenac sodium Injection
standard testing procedure of Fexofenadine and Phenylephrine suspension
standard testing procedure of Piroxicam Injection
STP of Fungal Diastase and Papain capsules
standard testing procedure PVC
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
Analysis method of aceclofenac
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
Analysis method of Suspension of Tribasic Calcium phosphate with vitamin D3 and Vitamin B12
Analysis method of Calcitriol with calcium citrate Suspension
Analysis method of Oxyclozanide and Fenbendazole Suspension
Analysis method of Oxyclozanide and Levamisole Suspension
Analysis method of Triclabendazole and Ivermectin Suspension
Analysis method of Itraconazole Solution
Analysis method of Levocetirizine Dihydrochloride syrup
Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
Analysis method of Ferrous Ascorbate Cyanocobalamin and Folic Acid Suspension
Analysis method of Ambroxol Hydrochloride Drops
Analysis method of Ferrous Ascorbate with Folic Acid suspension
Analysis method of Piracetam Syrup
Analysis method of Rafoxanide and Levamisole suspension
Analysis method of Zinc gluconate Syrup
Analysis method of Magaldrate Simethicone and Oxetacaine suspension
Analysis method of mefenamic acid and paracetamol suspension
Analysis method of Cholecalciferol Drops
Analysis method of Racecadotril suspension
Analysis method of Deflazacort Suspension
Analysis method of Montelukast sodium and levocetirizine Dihydrochloride Syrup
Analysis method of Iron and Folic Acid Syrup
Analysis method of Cyproheptadine Hydrochloride and Tricholine Citrate Syrup
Analysis method of Levofloxacin Hemihydrate Ornidazole and Vitamin E Solution
Analysis method of Albendazole and ivermectin in oral liquid