Standard testing procedure honey pure
Storage Requirements:
Store protected from light and moisture.
Sampling:
Sample from each container / bag, if the consignment is of 03or less than three containers / bags. If the number of containers / bags is more than 03and are up to 100, sample at randomly using the formula root (n) +1 where n is the number of containers/bags in the consignment. For the consignment of more than 100 containers/bags, sample additional containers/bags for every 100 containers/bags and thereafter. Collect a minimum of 5ml from each of the randomly selected containers/bags into individually no toxic, self sealing transparent polyethylene bearing ‘Sample For Analysis ‘Label kept in another transparent self sealing polythene bag. Afetr completion of sampling return rest sample on the same container. Collect control sample in Pet bottle/Glass bottle.
Quantity of Composite Sample:
1 x 100 ml
Description: Brownish clear viscous liquid.
Specific gravity: Not less than 1.25
Refractive Index: Not less than 1.4860and not more than 1.4965
Moisture content: Not more than 20.0%
Mineral content: 0.06 to 0.49g/100g.
Total water insoluble solids content: 0.01 to 0.03g/100g.
Description: Examine the individual samples by visually.
Reporting: Report as Complies/Does not comply.
Specific gravity (Relative Density): Not less than 1.017 and not more than 1.037.
Select a thoroughly clean and dry pycnometer. Calibrate the pycnometer by filling it with recently boiled and cooled water at 20º and weighing the contents. Assuming that the weight of 1 ml of water at 20º, calculate the capacity of the pycnometer. Adjust the temperature of the substance under examination, to about 20º and fill the pycnometer with it. Adjust the temperature of the filled pycnometer to 20º, remove any excess of the substance and weigh. Subtract the tare weight of the pycnometer from the filled weight of the pycnometer. Determine the weight per millilitre by dividing the weight in g, of the quantity of liquid which fills the pycnometer at the specified temperature, by the capacity expressed in ml, of the pycnometer at the same temperature.
Calculation:
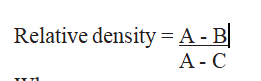
Where
C is mass in g of the specific gravity pycnometer with honey sample;
A is mass in g of the empty specific gravity pycnometer.
B is mass in g of the specific gravity pycnometer with water.
Reporting: Report as value.
Refractive Index: By Refractometer
Procedure
Determine the Refractometer reading of honey at 20°C and calculate the percentage of moisture from the values given in Table1. If the determination is made at a temperature other than 20 °C, correct the reading according to the Note in Table.
Reporting: Report as value.
Moisture content: By Refractometer
Procedure: Determine the Refractometer reading of honey at 20°C and calculate the percentage of moisture from the values given in Table1. If the determination is made at a temperature other than 20 °C, correct the reading according to the Note in Table.
Table1
Refractive indices, corresponding percent soluble solids, and percent moisture in extracted honey
| Refractive Index @ 20°C (Range) | Percent
Soluble Solids |
Percent
Moisture |
Refractive Index @ 20°C (Range) | Percent
Soluble Solids |
Percent
Moisture |
| 1.4817 – 1.4818 | 78.1 | 21.9 | 1.4930 – 1.4932 | 82.6 | 17.4 |
| 1.4819 – 1.4820 | 78.2 | 21.8 | 1.4933 – 1.4934 | 82.7 | 17.3 |
| 1.4821 – 1.4823 | 78.3 | 21.7 | 1.4935 – 1.4936 | 82.8 | 17.2 |
| 1.4824 – 1.4825 | 78.4 | 21.6 | 1.4937 – 1.4939 | 82.9 | 17.1 |
| 1.4826 – 1.4828 | 78.5 | 21.5 | 1.4940 – 1.4941 | 83.0 | 17.0 |
| 1.4829 – 1.4830 | 78.6 | 21.4 | 1.4942 – 1.4944 | 83.1 | 16.9 |
| 1.4831 – 1.4833 | 78.7 | 21.3 | 1.4945 – 1.4946 | 83.2 | 16.8 |
| 1.4834 – 1.4835 | 78.8 | 21.2 | 1.4947 – 1.4949 | 83.3 | 16.7 |
| 1.4836 – 1.4838 | 78.9 | 21.1 | 1.4950 – 1.4951 | 83.4 | 16.6 |
| 1.4839 – 14840 | 79.0 | 21.0 | 1.4952 – 1.4954 | 83.5 | 16.5 |
| 1.4841 – 1.4843 | 79.1 | 20.9 | 1.4955 – 1.4957 | 83.6 | 16.4 |
| 1.4844 – 1.4845 | 79.2 | 20.8 | 1.4958 – 1.4959 | 83.7 | 16.3 |
| 1.4846 – 1.4848 | 79.3 | 20.7 | 1.4960 – 1.4962 | 83.8 | 16.2 |
| 1.4849 – 1.4850 | 79.4 | 20.6 | 1.4963 – 1.4964 | 83.9 | 16.1 |
| 1.4851 – 1.4853 | 79.5 | 20.5 | 1.4965 – 1.4967 | 84.0 | 16.0 |
| 1.4854 – 1.4855 | 79.6 | 20.4 | 1.4968 – 1.4969 | 84.1 | 15.9 |
| 1.4856 – 1.4858 | 79.7 | 20.3 | 1.4970 – 1.4972 | 84.2 | 15.8 |
| 1.4859 – 1.4860 | 79.8 | 20.2 | 1.4973 – 1.4975 | 84.3 | 15.7 |
| 1.4861 – 1.4863 | 79.9 | 20.1 | 1.4976 – 1.4977 | 84.4 | 15.6 |
| 1.4864 – 1.4865 | 80.0 | 20.0 | 1.4978 – 1.4980 | 84.5 | 15.5 |
| 1.4866 – 1.4868 | 80.1 | 19.9 | 1.4981 – 1.4982 | 84.6 | 15.4 |
| 1.4869 – 1.4870 | 80.2 | 19.8 | 1.4983 – 1.4984 | 84.7 | 15.3 |
| 1.4871 – 1.4873 | 80.3 | 19.7 | 1.4985 – 1.4987 | 84.8 | 15.2 |
| 1.4874 – 1.4875 | 80.4 | 19.6 | 1.4988 – 1.4990 | 84.9 | 15.1 |
| 1.4876 – 1.4878 | 80.5 | 19.5 | 1.4991 – 1.4993 | 85.0 | 15.0 |
| 1.4879 – 1.4880 | 80.6 | 19.4 | 1.4994 – 1.4995 | 85.1 | 14.9 |
| 1.4881 – 1.4883 | 80.7 | 19.3 | 1.4996 – 1.4998 | 85.2 | 14.8 |
| 1.4884 – 1.4885 | 80.8 | 19.2 | 1.4999 – 1.5000 | 85.3 | 14.7 |
| 1.4886 – 1.4888 | 80.9 | 19.1 | 1.5001 – 1.5003 | 85.4 | 14.6 |
| 1.4889 – 1.4890 | 81.0 | 19.0 | 1.5004 – 1.5005 | 85.5 | 14.5 |
| 1.4891 – 1.4893 | 81.1 | 18.9 | 1.5006 – 1.5008 | 85.6 | 14.4 |
| 1.4894 – 1.4896 | 81.2 | 18.8 | 1.5009 – 1.5011 | 85.7 | 14.3 |
| 1.4897 – 1.4898 | 81.3 | 18.7 | 1.5012 – 1.5013 | 85.8 | 14.2 |
| 1.4899 – 1.4901 | 81.4 | 18.6 | 1.5014 – 1.5016 | 85.9 | 14.1 |
| 1.4902 – 1.4903 | 81.5 | 18.5 | 1.5017 – 1.5018 | 86.0 | 14.0 |
| 1.4904 – 1.4906 | 81.6 | 18.4 | 1.5019 – 1.5021 | 86.1 | 13.9 |
| 1.4907 – 1.4908 | 81.7 | 18.3 | 1.5022 – 1.5024 | 86.2 | 13.8 |
| 1.4909 – 1.4911 | 81.8 | 18.2 | 1.5025 – 1.5026 | 86.3 | 13.7 |
| 1.4912 – 1.4913 | 81.9 | 18.1 | 1.5027 – 1.5029 | 86.4 | 13.6 |
| 1.4914 – 1.4916 | 82.0 | 18.0 | 1.5030 – 1.5031 | 86.5 | 13.5 |
| 1.4917 – 1.4918 | 82.1 | 17.9 | 1.5032 – 1.5034 | 86.6 | 13.4 |
| 1.4919 – 1.4921 | 82.2 | 17.8 | 1.5035 – 1.5037 | 86.7 | 13.3 |
| 1.4922 – 1.4923 | 82.3 | 17.7 | 1.5038 – 1.5039 | 86.8 | 13.2 |
| 1.4924 – 1.4926 | 82.4 | 17.6 | 1.5040 – 1.5042 | 86.9 | 13.1 |
| 1.4927 – 1.4929 | 82.5 | 17.5 | 1.5043 – 1.5044 | 87.0 | 13.0 |
| 1.4912 – 1.4913 | 81.9 | 18.1 | 1.5027 – 1.5029 | 86.4 | 13.6 |
| 1.4914 – 1.4916 | 82.0 | 18.0 | 1.5030 – 1.5031 | 86.5 | 13.5 |
| 1.4917 – 1.4918 | 82.1 | 17.9 | 1.5032 – 1.5034 | 86.6 | 13.4 |
| 1.4919 – 1.4921 | 82.2 | 17.8 | 1.5035 – 1.5037 | 86.7 | 13.3 |
| 1.4922 – 1.4923 | 82.3 | 17.7 | 1.5038 – 1.5039 | 86.8 | 13.2 |
| 1.4924 – 1.4926 | 82.4 | 17.6 | 1.5040 – 1.5042 | 86.9 | 13.1 |
| 1.4927 – 1.4929 | 82.5 | 17.5 | 1.5043 – 1.5044 | 87.0 | 13.0 |
Reporting: Report as value in %.
Mineral content: By Ash
Procedure: Weight 5 g -10 g of the honey sample in an ignited and pre-weighed platinum or silica dish and gently
heat in a muffle furnace until the sample is black and dry. If necessary a few drops of olive oil may be added to prevent frothing. The sample is then ignited at 600 °C to constant weight. The sample is cooled in a desiccator and weighed.
Calculation and expression of results:
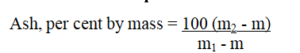
Where
M2 is the mass of the dish with the ash, g;
M is the mass of empty dish, g;
m1 is the mass of the dish with the material taken for the test, g.
The result is expressed as per cent ash (mass/mass)
Reporting: Report as value in %.
Total water insoluble solids content:
Preparation of test sample: About 20 g of honey is weighed accurately and dissolved in a suitable quantity of distilled water at 80 °C and mixed well.
Determination (gravimetric):
The test solution is filtered through a previously dried and weighed fine sintered glass crucible (pore size, 15 – 40 microns) and washed thoroughly with hot water (80°C) until free from sugars. The crucible is dried for one hour at 135 °C. Cooled and weighed to the nearest 0.1 mg.
Calculation and expression of results:
The result is expressed as per cent water insoluble solids (S), calculated as follows:
S = (% water insoluble solids) = X x100
W
Where,
X is the gain in weight of the crucible (weight of residue), (g)
W is the weight of the honey sample.
Reporting: Report as value in %.
Analysis of vitamin B1 B6 B2 Nicotinamide and sodium pentothenate Injection
Analysis for Nandrolone Decanoate injection
Analysis of Dicyclomine and Diclofenac sodium Injection
standard testing procedure of Fexofenadine and Phenylephrine suspension
standard testing procedure of Piroxicam Injection
STP of Fungal Diastase and Papain capsules
standard testing procedure PVC
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
Analysis method of aceclofenac
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
Analysis method of Suspension of Tribasic Calcium phosphate with vitamin D3 and Vitamin B12
Analysis method of Calcitriol with calcium citrate Suspension
Analysis method of Oxyclozanide and Fenbendazole Suspension
Analysis method of Oxyclozanide and Levamisole Suspension
Analysis method of Triclabendazole and Ivermectin Suspension
Analysis method of Itraconazole Solution
Analysis method of Levocetirizine Dihydrochloride syrup
Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
Analysis method of Ferrous Ascorbate Cyanocobalamin and Folic Acid Suspension
Analysis method of Ambroxol Hydrochloride Drops
Analysis method of Ferrous Ascorbate with Folic Acid suspension
Analysis method of Piracetam Syrup
Analysis method of Rafoxanide and Levamisole suspension
Analysis method of Zinc gluconate Syrup
Analysis method of Magaldrate Simethicone and Oxetacaine suspension
Analysis method of mefenamic acid and paracetamol suspension
Analysis method of Cholecalciferol Drops
Analysis method of Racecadotril suspension
Analysis method of Deflazacort Suspension
Analysis method of Montelukast sodium and levocetirizine Dihydrochloride Syrup
Analysis method of Iron and Folic Acid Syrup
Analysis method of Cyproheptadine Hydrochloride and Tricholine Citrate Syrup
Analysis method of Levofloxacin Hemihydrate Ornidazole and Vitamin E Solution
Analysis method of Albendazole and ivermectin in oral liquid