Standard Testing Procedure Tranexamic acid injection
1.0 OBJECTIVE
1.1 To lay down a procedure for Analysis of Tranexamic acid injection (1ml)
2.0 SCOPE
2.1 This procedure is applicable to the Analysis of Tranexamic acid (1ml) in quality control
3.0 RESPONSIBILITY
3.1 Q.C- Chemist
4.0 ACCOUNTABILITY
4.1 Head-Quality Assurance
5.0 Identification: To a volume containing 400 mg of Tranexamic acid, add 2 ml of ether, stir, add 5 ml methanol, stir again and allow
to crystalline. The crystals, after drying comply with the following test.
(A) Determine by I.R
(B) To 1ml of a 1 per w/v solution, add 1 ml of a 0.2 per cent w/v solution of Ninhydrine in ethanol and heat on water bath for 2 minute, a dark bluish violet colour is produced.
6.0 Description: Pour 10ml finish sample in Beaker and observed visually.
7.0 pH: Taken 40ml sample in beaker the pH electrode first with purified water followed by sample dip the electrode in sample and observe the pH.
8.0 Extractable volume: Measured the volume by 10 ml Measuring cylinder and determine the volume.
9.0 Particulate matter: Injections that are solutions, when examine under suitable conditions of visibility are clear and practically
free from particles that can be observed on visual inspection by the unaided eye.
10.0Sterility: – Injection comply with the test for sterility as per SOP
11.0 Bacterial endotoxins: –
NMT 35 Endotoxins unit/ml of Ondansetron as per SOP
12.0 Related substance: Determine by liquid chromatography.
Test solution: -Dilute the injection containing 1.0% w/v Tranexamic acid with water.
Reference solution (a) A 0.01 % w/v solution of the Tranexamic acid RS in water.
Reference solution (b) A 0.001 % w/v solution ml of ml 4-aminomethylbenzoic acid in water.
Mobile phase: – A mixture of a solution containing 11.0gm of anhydrous sodium dihydrogen orthophosphate in 500 ml of water,
add 5 ml of triethylamine and 1.4 gm. of sodium dodecyl sulphate, adjusted to pH 2.5 with 2M Orthophoshoric acid and
dilute to 600 ml with water and 400 volumes of methanol.
Chromatographic condition: –
Wavelength-220 nm
Flow rate 0.9 ml /mint.
Injection volume- 20 µl.
Column- C18 25cm x 4.6 mm 5µm bonded to porous silica
Inject reference solution(a) the test is not valid unless the column efficiency is not less than 2000 theoretical plates and the tailing factor is not more than 2.0.
1) Tranexamic acid Impurity A- Not more than 0.1%
2) Tranexamic acid Impurity B – Not more than 0.2%
3) Any secondary peak – Not more than 0.1%
4) Total impurities- Not more than 0.2%
13.0 Composition: –
Each 5 ml contains:
Tranexamic acid I.P. 500 mg.
14.0 Assay: – NLT 95.0% and NMT 105.0%
By Titration method:
To a volume containing 100 mg of Tranexamic acid, add 50 ml of water and adjust to pH 7.0 with sodium hydroxide or 0.1 M Hydrochloric acid.
Add 25 ml of formaldehyde solution, previously adjusted to pH 7.0 and 20 ml of sodium hydroxide and titrate with 0.1 M Hydrochloric acid
determining the end point potentiomertrically carry out a blank titration.
1ml of 0.1 M sodium hydroxide is equivalent to 15.72 mg of C8H15NO2.
Calculation: –
V= Volume (in ml) of Tranexamic acid Solution consumed in titration.
N = Actual normality of Hydrochloric acid.
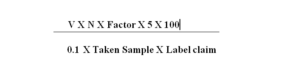
15.0 ABBREVIATION: –
| Sr. No. | Abbreviation used | Full form of abbreviation used |
| 1.0 | STP | Standard Testing Procedure |
| 2.0 | QA | Quality assurance |
| 3.0 | STD | Standard |
| 4.0 | SPL | Sample |
16.0 REFERENCE: –
| Sr. No. | Reference Title |
| 01 | I.P. |
Analysis of vitamin B1 B6 B2 Nicotinamide and sodium pentothenate Injection
Analysis for Nandrolone Decanoate injection
Analysis of Dicyclomine and Diclofenac sodium Injection
standard testing procedure of Fexofenadine and Phenylephrine suspension
standard testing procedure of Piroxicam Injection
STP of Fungal Diastase and Papain capsules
standard testing procedure PVC
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
Analysis method of aceclofenac
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
Analysis method of Suspension of Tribasic Calcium phosphate with vitamin D3 and Vitamin B12
Analysis method of Calcitriol with calcium citrate Suspension
Analysis method of Oxyclozanide and Fenbendazole Suspension
Analysis method of Oxyclozanide and Levamisole Suspension
Analysis method of Triclabendazole and Ivermectin Suspension
Analysis method of Itraconazole Solution
Analysis method of Levocetirizine Dihydrochloride syrup
Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
Analysis method of Ferrous Ascorbate Cyanocobalamin and Folic Acid Suspension
Analysis method of Ambroxol Hydrochloride Drops
Analysis method of Ferrous Ascorbate with Folic Acid suspension
Analysis method of Piracetam Syrup
Analysis method of Rafoxanide and Levamisole suspension
Analysis method of Zinc gluconate Syrup
Analysis method of Magaldrate Simethicone and Oxetacaine suspension
Analysis method of mefenamic acid and paracetamol suspension
Analysis method of Cholecalciferol Drops
Analysis method of Racecadotril suspension
Analysis method of Deflazacort Suspension
Analysis method of Montelukast sodium and levocetirizine Dihydrochloride Syrup
Analysis method of Iron and Folic Acid Syrup
Analysis method of Cyproheptadine Hydrochloride and Tricholine Citrate Syrup
Analysis method of Levofloxacin Hemihydrate Ornidazole and Vitamin E Solution
Analysis method of Albendazole and ivermectin in oral liquid