STP of Fungal Diastase and Papain capsules
Description : Blue/white coloured ‘2’ size capsule with cream coloured powder.
Weight of 20 Capsules : 7.3 gm. Limits +/- 7.5%
Average weight of Capsule : 0.365 gm. Limits +/- 7.5%
Average net content of Capsule : 0.300 gm. Limits +/- 7.5%
Disintegration : NMT 30 Minutes.
Each Capsule Contains Lable Claim Result Limits
Fungal Diastase (1:800) I.P. 100 mg NLT 90 %
(Fungal Diastase derived from aspergillus oryzae. Disests not less than 80 gms of cooked starch.)
Papain I.P. 60 mg NLT 90 %
Fungal Diastase
Reagents:
1. Freshly preparation normal saline solution (0.9%), PH 5.0
2. Soluble starch having about 15% moisture content – BDH grade starch is most sutable.
3. Buffer solution, PH 5.0 : Weigh accurately 2.05 gm of Sodium Acetate, dissolve in sufficient
quantity of water and make the volume to 250 ml. When 5.9 ml of 0.1 M Acetic Acid is mixed
with 14 ml of 0.1 M Sodium Acetate, solution will attain PH 5.0
4. Iodine Solution : 1.276 gm of Iodine and dissolve in 15 ml of water containing 4 gms.
Of Potassium Iodide and make the volume to 100 ml. Standardise this solution to get a solution of exactly 0.1 M.
5. Iodine Solution, 0.02 M : Dilute 20 ml of the Stock solution (0.1 M) with 80 ml of water
to obtain 0.02 M Iodine solution. Prapare 0.02 M Iodine solution just before use, and use 1 drop of this solution, per tube.
6. 10 M Hydrochloride, to arrest the enzyme reaction.
7. Substrate : Weigh 200 mg of soluble starch accurately ( on Dry Weight basis) and transfer
into 200 ml measuring flask with little quantity of water. Boil the suspension till gets into solution,
constant shaking the flask while boiling. Cool under tap water, add 5.9 ml of 0.1 M Acetic Acid , 14 ml of 0.1 M Sodium
Acetate and finally make the volume to 200 ml with normal saline, PH 5.0. Dispense this solution in 5 ml
aliquot to each to each tube. Thus each tube contains 5 mg of Soluble starch.
Dilution of Sample : All dilutions are to be made with normal saline solution, PH 5.0.
The Sample is diluted so that the final solution containing 5 mcg/ml ( 1:2000).
This Solution is used as test enzyme solution in greaded amounts of 0.1 ml to 1.0 ml per tube.
Procedure:
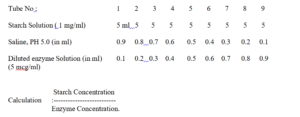
Despence substrate (Starch Solution) and saline solution as shown in the following table, and pre- Incubate the tubes for 5 min in a water- bath at 370 C (+/-0.50 C). Then, add greaded amount of diluted enzyme solution, shake to mix well and incubate for 1 hour at 370 C. At the end of incubation period, Stop the enzyme action by adding 5 drops of 10 M Hydrochloric Acid. Shake the tube well after adding Hydrochloric Acid. Add to each tube 1 drop of 0.002 M Iodine and shake well. Note the change of colour in the tubes. With Increasing concentration of enzyme, there will be fall in the intensity of the colour from light purple, red to colourless. The tube showing no colour on the addition of iodine is to be taken as the end point.
It is assumed that amount of enzyme present in the tube showing no colour on the addition of iodine solution hasfully digested the quantity of starch present,
Papain:
Reagents:
1.Cystein Hydrochloride : Dissolve 0.5 gms. Of Cystein Hydrochloride accurately weighed in 10 ml of water, adjust
to PH 7.0 with solution of Sodium Hydroxide. It should freshly prepared.
2. Casein Solution : Dissolve 4.0 gms. Of purified Casein accurately weighed by shaking with 90 ml of water.
Adjust to PH 7.0 with 1 M Sodium Hydroxide and dilute to 100 ml with water.
Assay preparation : Take 100 mg of sample add 10 ml of Cystein Hydrochloride and dilute to 100 ml of water to get 1 mg/ml .
Procedure:
To 10 ml of water in each of two flasks, add 15 ml accurately measured solution of casein and maintain at 600 C by heating
on water-bath. To the first flask, add 25 ml of accurately measured assay preparation and to the second flask,
add 25 ml accurately measured same assay solution previously boiled for 3 minutes and cooled.
Maintain the solution at 600 C for 30 minutes, cool rapidly to room temparature and add to each
flask 10 ml of solution of formaldehyde previously neutralised to Phenolpthalene. Titrate both solutions
with 0.1 M Sodium Hydroxide to PH 8. The difference between the two titrations is not less than 4.5 ml.
Analysis of vitamin B1 B6 B2 Nicotinamide and sodium pentothenate Injection
Analysis for Nandrolone Decanoate injection
Analysis of Dicyclomine and Diclofenac sodium Injection
standard testing procedure of Fexofenadine and Phenylephrine suspension
standard testing procedure of Piroxicam Injection
STP of Fungal Diastase and Papain capsules
standard testing procedure PVC
standard testing procedure glass ampoule
Standard testing procedure of Iron Sucrose Injection
Standard testing procedure lactose
Standard testing procedure mefenamic acid
standard testing procedure domperidone
standard testing procedure flavour mixed fruit
standard testing procedure dicyclomine hydrochloride
Standard testing procedure honey pure
standard testing procedure dextromethorphan hydrobromide
standard procedure of levocarnitine injection
Analysis of Ivermectin Suspension
standard testing procedure artemether injection
standard testing procedure artemether injection
standard testing procedure Carbocisteine syrup
standard testing procedure Phytomenadione injection
standard testing procedure serratiopeptidase
standard testing procedure starch IP
standard testing procedure sucrose refined sugar
standard testing procedure titanium dioxide
standard testing procedure tramadol hydrochloride
standard testing procedure zinc sulphate
standard testing procedure croscarmellose sodium
standard testing procedure colour erythrosine supra
standard testing procedure magnesium hydroxide
standard testing procedure diclofenac sodium
standard testing procedure dibasic calcium phosphate
standard testing procedure cyanocobalamin
standard testing procedure cholecalciferol
standard testing procedure Calcium carbonate oyster shell powder
standard test procedure Calcium Citrate
standard testing procedure Bronopol
standard testing procedure Bromhexine Hydrochloride
Standard Testing Procedure diclofenac sodium injection
Standard Testing Procedure Drotaverine Hydrochloride injection
Standard Testing Procedure Tranexamic acid injection
standard test procedure paracetamol infusion
standard test procedure ofloxacin and ornidazole infusion
standard test procedure ornidazole injection
standard test procedure Ondansetron injection
standard test procedure dextrose injection
standard test procedure ciprofloxacin injection
STP and analysis method of Ammonium Chloride
Analysis method of aceclofenac
analysis method of Losartan Potassium and Hydrochlorothiazide
analysis method of Linezolid Dry Syrup
analysis method of Drotaverine Hydrochloride and Mefenamic acid
Analysis method of Ceftriaxone Sodium and Sulbactam sodium Injection
analysis method of Cefepime and Tazobactam Injection
Analysis method of Hydroquinone Cream
Analysis method of Tacrolimus Ointment
Analysis method of Terbinafine HCL Cream
Analysis method of Mometasone Furoate and Fusidic Acid Cream
Analysis method of Disodium Hydrogen Citrate Syrup
Analysis method of Hydroquinone with Tretinoin Cream
Analysis method of Hydroquinone Tretinoin and Mometasone Furoate Cream
Analysis method of Sertaconazole Nitrate Cream
Analysis method of Halobetasol Propionate Cream
Analysis method of Povidone Iodine with Ornidazole Ointment
Analysis method of Eberconazole Cream
Analysis method of Luliconazole Cream
Analysis method of Fluconazole Gel
Analysis method of Ketoconazole Cream
Analysis method of Salbutamol and Choline theophyllinate Syrup
Analysis method of Methylcobalamin Injection
Analysis method of Piroxicam and paracetamol Injection
Analysis method of Alpha Beta Arteether Injection
Analysis method of Enrofloxacin Suspension
Analysis method of Levetiracetam Syrup
Analysis method of Sucralfate suspension
Analysis method of Sucralfate and Oxetacaine Suspension
Analysis method of Quinine Sulphate Suspension
Analysis method of Calcium Carbonate vitamin D3 Zinc Gluconate and Magnesium hydroxide suspension
Analysis method of Suspension of Tribasic Calcium phosphate with vitamin D3 and Vitamin B12
Analysis method of Calcitriol with calcium citrate Suspension
Analysis method of Oxyclozanide and Fenbendazole Suspension
Analysis method of Oxyclozanide and Levamisole Suspension
Analysis method of Triclabendazole and Ivermectin Suspension
Analysis method of Itraconazole Solution
Analysis method of Levocetirizine Dihydrochloride syrup
Analysis method of Iron Calcium Vitamin D3 Folic Acid Vitamin B12 Suspension
Analysis method of Ferrous Ascorbate Cyanocobalamin and Folic Acid Suspension
Analysis method of Ambroxol Hydrochloride Drops
Analysis method of Ferrous Ascorbate with Folic Acid suspension
Analysis method of Piracetam Syrup
Analysis method of Rafoxanide and Levamisole suspension
Analysis method of Zinc gluconate Syrup
Analysis method of Magaldrate Simethicone and Oxetacaine suspension
Analysis method of mefenamic acid and paracetamol suspension
Analysis method of Cholecalciferol Drops
Analysis method of Racecadotril suspension
Analysis method of Deflazacort Suspension
Analysis method of Montelukast sodium and levocetirizine Dihydrochloride Syrup
Analysis method of Iron and Folic Acid Syrup
Analysis method of Cyproheptadine Hydrochloride and Tricholine Citrate Syrup
Analysis method of Levofloxacin Hemihydrate Ornidazole and Vitamin E Solution
Analysis method of Albendazole and ivermectin in oral liquid