Microbial monitoring of Drain points
1.0 Objective
To lay down a procedure to describe the steps to be followed to monitor drain points for microbial load.
2.0 Scope
This Standard Operating Procedure is applicable for monitoring the microbiological load at drain points to be followed at formulation plant of abc company.
3.0 Responsibility
3.1 Officer / Executive Quality Control are responsible for monitoring of drain points for microbial load.
3.2 Head Quality Control is responsible for compliance of this SOP.
4.0 Abbreviations and Definitions
IPA : Isopropyl Alcohol
Ml : Milliliter
Cm : Centimeter
SCDM : Soyabean Casein Digest Medium
Hrs : Hours
ºC : Degree Centigrade
5.0 Procedure
5.1 Preparation of media and glassware
5.1.1 Prepare the media as per instruction.
5.1.2 Sterilize the media flasks and test tubes containing 10ml normal saline, Petri plate & accessories.
5.1.3 After completion of sterilization, remove the media and glass Petri plates, test tubes containing 10 ml
normal saline from the autoclave and cool it. Glass Petri plates dried in Hot air oven.
5.1.4 Maintain the sterilized Soyabean Casein Digest agar at 55°C.
5.2 Swab Sampling
5.2.1 Place all marked test tubes containing 10 ml normal saline in carrier box and transfer to sampling area.
5.2.2 Remove sterile swab carefully and place in the test tube containing sterile 10-ml normal saline
carefully remove the swab from the test tube. Select the surface to be tested & sanitize with 70 % IPA after sampling.
5.2.3 Take the swabs from select surfaces area (25 cm2) of each drain point.
5.2.4 After each swabbing, collect the swab test tubes corresponding to the location and transfer the samples
to Microbiology lab for analysis.
5.2.5 Under aseptic conditions, vortex the normal saline test-tube for proper extraction, remove a swab.
Using a micropipette with sterile tip, remove 1 ml of the sample for total viable aerobic counts and 1 ml for
pathogen transfer into SCDM.
5.2.6 Label the Petri plate, with sampling location, media lot Number and date. Incubate the
Soyabean Casein Digest agar plates at 30°C to 35°C for 72 hrs. & followed by 20°C to 25°C for 48 hrs.
5.2.7 Keep Negative control for each lot of medium prepared. Note down the details of media preparation
and growth promotion test.
5.2.8 Incubation the SCDM for 24 to 48 hrs and carry out testing for detection of pathogens.
(Escherichia coli, Salmonella spp, Staphylococcus aureus and Pseudomonas aeruugionsa).
5.2.9 Pathogen testing should be performed.
5.2.10 Limit for total bacterial count is 200cfu/swab and for total mould/yeast count <10cfu/swab
and pathogen should be absent.
5.2.11 Frequency of monitoring every month.
6.0 Forms and Records
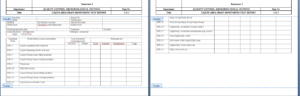
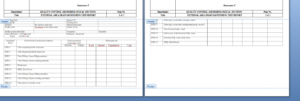
6.1 Liquid Area Drain Monitoring Test Report – Annexure-1
6.2 External Area Drain Monitoring Test Report – Annexure-2
6.3 Tablet and Capsule Area Drain Monitoring Test Report – Annexure-3
7.0 Distribution
7.1 Master copy – Documentation Cell (Quality Assurance)
7.2 Control copy – Quality Control
8.0 History
| Revision Number | Details For Change |
Reason for Revision |
| 00 | New SOP | NA |

sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware
Preparation Filtration Usage and Destruction of Disinfectant Solution
Entry Exit and gowning Procedure in Microbiology Laboratory
Safety measures to be followed in microbiology laboratory
Growth Promotion Test of Media
Preservation and Maintenance of Microbial Strain
Entry and Exit in Microbiology Sterile Area
Entry and Exit in Microbiology Limit testing area
Water sampling of raw water purified water WFI and PSG in microbiology Lab