Analysis of water for microbial load in microbiology lab
1.0 OBJECTIVE
1.1 To lay down the procedure for analysis of water sample for estimation of the number of viable aerobic micro- organisms
present & for the detection of Pathogenic microbial species.
2.0 SCOPE
2.1 This procedure is applicable to the analysis of water for microbial load in microbiology lab
3.0 RESPONSIBILITY
3.1 Microbiologist QC
4.0 ACCOUNTABILITY
4.1 Q. C. Manager
5.0 PROCEDURE
5.1 PRECAUTIONS
5.1.1 Water sampling should be done in sterile screw cap bottles.
5.1.2 Sampled the water aseptically in sampling bottle.
5.1.3 Media should be fresh for use in test.
5.2 MATERIALS REQUIRED
5.2.1 Soyabean Casein Digest Medium, R-2A media, Peptone water, Sterile Petri plates, test tubes,

Micropipette (1ml), Micro tips (1ml), Filtration assemblies, Sterile Gridded nitrocellulose
Filter paper (0.45 μ), Sterile forceps, Sterile scissor, Filtered 70%IPA, Selective media as per requirement.
5.3 EQUIPMENTS REQUIRED
5.3.1 HLAF, Autoclave, Incubators, Colony counter, Inoculating loop.
5.4 Methodology
5.5 Total Aerobic Microbial Count
5.6 Plate count method: (Potable Water)
5.6.1 Take 1.0 ml of the sample in sterilized Petri plate.
5.6.2 Pour 15.0 to 20.0 ml of autoclaved and cooled to 45°C of R2A agar medium.
5.6.3 Mix the contents of the Petri plate by rotating it gently and allow the media to solidify.
5.6.4 Invert the Petri plates and incubate in BOD incubator at 30˚C to 35˚C for 5 days for bacterial
and fungal count.
5.6.5 After incubation examine the Petri plates for growth and count the number of colonies by using
Colony counter.
5.7 Membrane filtration method ( WFI/PSG and Purified Water)
5.7.1 Filtered 100ml of WFI /PSG& 1ml Purified water sample inoculate to100ml sterile peptone, from
water sampling bottle.
5.7.2 Remove butter paper from filtration assembly and silicon tubing under HLAF.
5.7.3 Connect filtration assembly to the filtration flask and vacuum pump with the help of silicon tubing.
5.7.4 Take out the sterile 0.45μ membrane filter from the individual pack and place the filter paper
sandwiched between filtration cup & filtration receptacle with the help of sterile forceps.
5.7.5 Open the lid of filtration cup filter the approx. 15-20ml sterile peptone water for rinsing of filter paper.
5.7.6 Switch “ON” the vacuum pump. Open the lid of filtration cup & pour the content of sampling bottle
into the filtration cup.
5.7.7 Switch off the vacuum pump and remove the SS receptacle from the holder base aseptically.
5.7.8 By means of sterile forceps transfer the filter paper on the R2A agar plate aseptically.
5.7.9 Incubate the plate at 30˚C to 35˚C for 5 days.
5.7.10 After Completion of incubation period, observe the colonies and count by using colony Counter.
5.8 Pathogens testing
5.8.1 Filter the 100ml of water sample and transfer the filter paper very carefully in 100ml Soybean Casein
Digest Medium, shake gently and incubate at 30°C -35°C for 18 to 24 hrs. After incubation examine the tube
If growth is present mix by gentle shaking. Further proceed for the specified test.
5.9 Test for E.coli
5.9.1 Primary Test:
Shake the tube, transfer 1.0 ml of soyabean casein digest broth into bottle containing 100ml of
MacConkey broth and incubate at 42 C to 44˚C for 24 to 48 hrs.
5.9.2 Secondary Test:
Subculture on the surface of MacConkey agar plate and incubate at 30°C to 35°C for 18 to 72 hrs. If
Appearance of pinkish red color colonies on MacConkey agar indicates the presence of E.coli.
MORPHOLOGICAL CHARACTERITICS OF E.coli SPECIES ON
| MEDIUM | DESCRIPTION OFCOLONY |
| Mac Conkey agar
|
Pinkish red colour colonies may have surrounding zone of precipitated bile
Growth present
|
5.10 Test for Salmonella
5.10.1 Filter the 100ml of water sample and transfer the filter paper very carefully in 100ml Soybean Casein
Digest Medium, shake gently and incubate at 30°C to 35°C 18 to 24 hrs. After incubation examine
the tube. If growth is present mix by gentle shaking. Further proceed for the specified test.
5.10.2Primary Test:
Add 0.1 ml of enrichment culture transfer test tube containing 10 ml Rappaport Vassiliadis
Salmonella Enrichment Broth and incubate at 30˚C to 35˚C for 24 to 48 hrs. After incubation
the tube subculture on the Xylose Lysine Deoxycholate Agar and incubate at 30˚C to 35˚C for 24 to
48 hrs. If red colonies with or without black centers indicate the presence of salmonella.
MORPHOLOGICAL CHARACTERITICS OF SALMONELLA SPECIES ON
| MEDIUM | DESCRIPTION OF COLONY |
| Xylose Lysine Deoxycholate Agar | Red with or without black centers |
5.11 Test for Pseudomonas aeruginosa
5.11.1Filter the 100ml of water sample and transfer the filter paper very carefully in 100ml.Soybean Casein Digest Medium,
shake gently and incubate at 30°C to 35°C 18 to 24 hrs. After incubation examine the tube. If growth is present mix by gentle shaking.
Further proceed for the specified test.
5.11.2Primary Test: Streak one loop full of the enrichment culture on the surface of Cetrimide agar plate, and incubate the plates in
inverted position at 30 to 35 ˚C for 18 to 72 hrs. Greenish color colonies indicate the possibility of Pseudomonas aeruginosa
MORPHOLOGICAL CHARACTERITICS OF SALMONELLA SPECIES ON
| Medium | Characteristics colonial morphology | Fluorescence in UV light |
| Cetrimide agar | Generally greenish | Greenish |
5.12 Test for Staphylococcus aureus
5.12.1Filter the 100ml of water sample and transfer the filter paper very carefully in 100ml
Soybean Casein Digest Medium, shake gently and incubate at 30°C to 35°C for 18 to 24 hrs. After
incubation examine the tube. If growth is present mix by gentle shaking. Further proceed for the
specified test.
5.12.2Primary Test: Streak one loop full of the enrichment culture on the surface of Mannitol Salt agar incubate the
plates inverted position at 30°C to 35°C for 18 to 72 hrs. Yellow or white colonies with yellow zones indicate the
possibility of presence of Staphylococcus aureus.
.
MORPHOLOGICAL CHARACTERITICS OF SALMONELLA SPECIES ON
| MEDIUM | DESCRIPTION OF COLONY |
| Mannitol Salt agar | Yellow colonies with yellow zones |
5.13 FREQUENCY
Daily
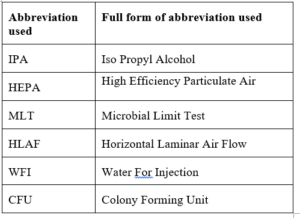
6.0 ABBREVIATIONS
sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
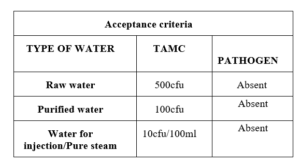
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware