Media received and preparation Management
1.0 OBJECTIVE
Lay down the procedure for Media received and preparation Management
2.0 SCOPE
This procedure is applicable for Lab media management at ……
3.0 RESPONSIBILITY
• Department microbiologist, officer and executive shall be responsible to follow the procedure of preparation and usage of sterile media as per SOP.
• Section head shall be responsible to ensure the overall compliance of the SOP.
4.0 ACCOUNTABILITY
• Department Head
• Head Quality / QA
5.0 PROCEDURE
5.1 Receipt of Media :
5.1.1 Microbiological Media shall be procured only from authorized dealer.
5.1.2 On receipt of the media check the Manufacturing Date, Expiry Date, and Batch Number of the media and integrity of the container.
5.1.3 Get the manufacturer Certificate of Analysis copy for the particular lot received.
5.1.4 If certificate is available on manufacturer website then Certificate can also get from their website.
5.1.5 Carefully read the instructions printed on the label of dehydrated culture media containers. Check the composition of the medium and the direction of use.
5.1.6 If any discrepancy found, return the media back to store.
5.1.7 An inventory record for all culture media shall be prepared and maintained as per Format No “Newly Media receipt, issuance and
reconciliation record”.
5.1.8 Media lot no shall be remain same as per manufacturer, if same lot is received again then it shall be denoted by one alphabet started from A to Z. ( If media lot no is 12345, and same media received again then media lot no hall be denoted as : 12345-A)
5.1.9 Affix a label on each media container as per format no
5.2 Usage and Qualification of newly received media :
5.2.1 Ensure that the media stock shall be used on first in first out basis.
5.2.2 Update the stock consumption record whenever media is withdrawn for routine use as per format no. “Newly Media receipt, issuance and reconciliation record”
5.2.2.1 Take the media sample from the one containers of same lot number and take prescribed quantity as per manufacturer instructions.
5.2.3 During qualification of the media ensure that the dehydrated media powder have the following physical characteristics :
Free Flowing
Uniform color
Absence of lumps
5.2.4 Do not use the media and reject the container, if not satisfied with reference to the above-mentioned characteristics.
5.2.4.1 Carry out the growth promotion test of each lot of new receipt culture media as per SOP No. “Media growth promotion, inhibition and sterility check” and record the observations of media in Format No. for “Qualification Record of Newly Received Media”
5.2.4.2 The new container of any media of new lot / batch shall not used for analysis until its growth promotion test complies.
5.2.5 After completion of GPT of the media, update the status and GPT ref no. of approved media as per Format No. “Newly Media receipt, issuance and reconciliation record” and update the label of the media container as per format no.: “Specimen label for received new container”
5.3 Storage:
5.3.1 Microbiological media shall be stored /used up to the expiry date specified on the manufacturer label or certificate of analysis.
5.3.2 All media containers shall be stored at defined storage condition recommended by the manufacturer, in case if storage temperature not recommended then the media shall be stored below 30°C in a dry place
5.3.3 Expired media shall be destroyed as per approved procedure mention in SOP “decontamination and disposal of microbial waste”
5.3.4 If stored culture media showing any change in appearance or any sign of visible microbial contamination or cracks or dehydration shall be discarded.
5.4 Shelf life of opened Media container
5.4.1 Media shall not be used after the expiry date assigned by the manufacturer. However each container of media opened shall be assigned a retesting date.
5.4.2 Retest date of GPT passed media is six month, if not used within six month the media shall be subjected to GPT every six month.
5.4.3 On half yearly basis (at frequency of ±1 month) media container shall be re-test for growth promotion / indicative / inhibitory properties (as applicable) as per routine testing procedure SOP on growth promotion inhibitory, indicative and sterility check.
5.4.4 Media shall be used after 6 month period, if it passes the above mentioned test and at the time retesting period shall be extended for another 6 month or up to the shelf life of media more (whichever is less).
5.5 Media Preparation (using readymade dehydrated media from hi-media or from other vendor):
5.5.1 Weigh the required quantity of respective culture media
5.5.2 Take a cleaned conical flask (select suitable flask / bottle having sufficient capacity to handle the media or as per load pattern validated during autoclave qualification) and transfer the weighed quantity of culture media in it.
5.5.3 If required add β- lactamase as per SOP no. “Usage of Beta-lactamase”.
5.5.4 Add sufficient quantity of purified water in conical flask/bottle and swirl the flask to dissolve the media. Then add purified water to make up final volume
5.5.5 If required, heat the flask on water bath/ Hot plate to dissolve the media with continuous shaking to avoid overheating.
5.5.6 Close the flasks with autoclavable plastic caps/ non-absorbent cotton plugs and wrap the flask with aluminum foil / butter paper (in case of cotton plug).
5.5.7 Each media prepared shall be labeled with media abbreviation (as per format no.: “”List of media /reagent”), media preparation lot number and sign on the media flask/bottle.
5.5.8 Media Preparation record shall be maintained as per current version of format no.:
5.6 Preparation of Media Petri plates [90mm diameter] Contact plates [55-65mm diameter]
5.6.1.1 After sterilization, media shall be unloaded in autoclave unloading area and transferred to respective area through dynamic passbox.
5.6.1.2 The sterilized outer wrap of the Petri plates shall be removed in the laminar air flow station.
5.6.1.3 The Petri plates shall be arranged in the laminar work station in such a way that it does not obstruct the laminarity of the air flow.
5.6.1.4 Media shall be poured onto the sterilized Petri plates either directly from the sterilized media flask or if the flask is of higher capacity, the media shall be dispensed in small sterilized beakers and then poured separately.
5.6.1.5 20-25mL of the media shall be poured into the 90mm Petri dishes and 10-12mL of media shall be poured in 55mm Petri dishes.
5.6.1.6 The media shall be dispensed at the temperature of not more than 45oC to avoid condensation in the cover of the Petri plates and without any air bubbles.
5.6.1.7 Sterilized culture media shall be shall be melted only once in water bath /hot plate after their sterilization and solidification
5.6.1.8 Molten agar medium also be held in a water bath at a temperature of 40-55oC for not more than 8 hours.
5.6.1.9 After media pouring, the Petri plates shall be covered with lid and allowed to solidify in the laminar air flow station.
5.6.1.10 After the media is solidified in the Petri plates, “Media preparation no” and “Media abbreviation” (as per format no. “List of media/reagents”) shall be marked on the bottom position of the media plates.
5.6.1.11 Whenever possible mark all the plates at the bottom of the plates otherwise mark all the details on the first plate of the stack during incubation in the incubator or SS container shall also be used for pre-incubation in such case mention all the details on the SS container using a marker pen.
5.6.1.12 Take one Petri plate /test tube containing media to check the pH (after sterilization) by using flat bottom electrode, and check the pH of the media.
5.6.1.13 Update the details of pH as per format no. “Media preparation record”
5.6.1.14 Incubate the plates for 24-48 hours at 30°C to 35°C for pre incubation and then store at 20˚C to 25˚C maintain the “Media reconciliation record” as per format No.:
Note:
• If media is not used in the same day then sterilized media shall be incubate at 30-35 o C in incubator for 24 -48 hrs. After completion of the incubation period, check the medium visually for any growth or turbidity. If any growth or turbidity observed in media, discard the media as per SOP No.QC04-008 “Decontamination and disposal microbial waste”.
• If GPT is not performed on the same date, then GPT of the prepared media shall be performed on next working day.
5.7 Preparation of media slants
5.7.1.1 Take a measuring cylinder or beaker and dispense sufficient quantity of the media (ensure to achieve minimum 1 inch butt when placed in slanting position) in the test tube plug the tubes with non-absorbent cotton / screw the cap if applicable.
5.7.1.2 Sterilize the test tubes containing media, in horizontal autoclave as per SOP No.: Operation, Cleaning and maintenance of Horizontal Autoclave.
5.7.1.3 Unload the tubes from the autoclave and place in slanting position to achieve a butt (minimum 1 inch).
5.7.1.4 Ensure that the top of the slant shall not touch the cotton plug/cap of the test tube then allow the slants to solidify.
5.7.2 Alternate method for slant preparation:
5.7.2.1 Sterilize the test tubes containing media, in horizontal autoclave as per SOP No.: Operation, Cleaning and maintenance of Horizontal Autoclave.
5.7.2.2 After sterilization, take a sterilized measuring cylinder or beaker and dispense sufficient quantity of the media (ensure to achieve minimum 1 inch butt when placed in slanting position) in the sterile test tube (under laminar air flow) and plug the tubes with non-absorbent cotton / screw the cap if applicable.
5.7.2.3 Place the test tubes in slanting position to achieve a butt (minimum 1 inch).
5.7.2.4 After incubation check the slants visually for any contamination.
5.7.2.5 If any contamination / growth observed in slants, discard the same.
5.7.2.6 Mark the slant tubes properly with the “Media abbreviation”.
5.7.2.7 Store the pre-incubated slants at 20°C to25°C in incubator.
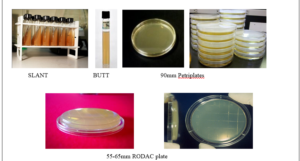
5.8 Preparation of liquid media
5.8.1.1 Follow the steps as mention in clause no 5.5.1 to 5.5.5.
5.8.1.2 Dispensed the media before sterilization in respective tube.
5.8.1.3 Sterilized the media as per SOP no.: “Operation and Maintenance of horizontal autoclave”
5.8.1.4 After sterilization media shall be unloaded in unloading area and transferred to incubation room for pre-incubation through dynamic pass box.
5.8.2 For preparation of 100 ml normal saline weigh and transfer 900 mg of NaCl (Sodium chloride) to flask/bottle containing 100 ml purified water to have 0.9% solution & dispense to tubes as per requirement or directly sterilize the flask/bottle as per load pattern. The required volume of solution in same ratio can be prepared for use. Sterilized normal saline shall be stored at 20-25˚C.
5.8.3 Pre incubate the prepared broth media / tubes at 30˚C – 35˚C for 24-48 hours and then store at 20˚C to 25˚C.
5.8.4 Media reconciliation shall be recorded in the current version of the format “Media Reconciliation record”
5.8.5 Perform Growth promotion test for all prepared media as per SOP no.: “Media growth promotion, inhibition, indicative and sterility check”.
5.8.6 Prepared media plates shall be used within a period of 10 days from the date of preparation when stored at temperature 20-25oC or as per validated period.
5.8.7 Liquid media tube shall be used within a period of 20 days from the date of preparation when stored at temperature 20-25̊C or as per validated period.
5.8.8 Before use each type of media shall be checked visually for any change in appearance, crack of solid agar media, turbidity in broth media, obnoxious smell etc.
Note: Media pre-incubation and GPT test shall be carried out before or in parallel with the analysis.
5.9 Assigning Media Preparation No.
5.9.1 Each media prepared shall be assigned a media preparation number.
5.9.2 The media preparation lot no. shall be assigned as PDDMMYY-XXX. Where P stands for preparation, DD stands for day, mm stands for month and YY stands for year. XXX stands for sequential no starting from 001 on each day. e.g. first media prepared on 04/12/19 shall be assigned media preparation no. 001, second media prepared on shall be assigned as
5.10 Disposal of Media.
5.10.1 If any growth observed in petri-plates/tubes during pre-incubation, discard the same as per SOP No- “Decontamination and disposal of microbial waste” and mention the same in remark column of format no Title “Media Reconciliation record” as discarded.
5.10.1.1 Discard the used or unused media, media plates/tubes after it’s due as per SOP no.: “Decontamination and disposal of microbial waste”
5.10.1.2 Any spillage shall be handled as per SOP no.: “Handling of spillage in Microbiology Laboratory”.
6.0 FORMATS
| Sr. No. | Title of the Format |
| 1. | Media preparation Record. |
| 2. | Flow Chart |
| 3. | List of Media / Reagents |
| 4. | Newly Media Receipt, Issuance And Reconciliation Record |
| 5. | Specimen label for received new container |
| 6. | Media Reconciliation record |
7.0 REFERENCES
PIC/S PART I PE 009-14 “Guide to Good Manufacturing Practice for medicinal products”
USP <71> “Sterility Tests”
USP <1117> “Microbiological Best Laboratory Practices”
8.0 ABBREVIATIONS
| SOP | Standard Operating Procedure | No. | Number |
| QA | Quality Assurance | F | Format |
| QC | Quality Control | oC | Degree centigrade |
| % | Percent | ml | Mille liter |
| OSD | Oral Solid Dosage | DPI | Dry Powder Injection |
| RODAC | Replicate organism Detection and count | M | Molarity |
op for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware