sop for Inoculum Preparation
1.0 OBJECTIVE
1.1 To lay down a procedure for Culture Suspension (Inoculum) preparation to determine 10 to 100 cfu/ml by Serial Dilution Method.
2.0 SCOPE
2.2 This procedure is applicable to the procedure of Culture Suspension (Inoculum) preparation of 10-100 cfu/ml which is used for Media Growth Promotion Test and various Microbiological test method validation studies in Microbiology lab
3.0 RESPONSIBILITY
3.3 Microbiologist QC
4.0 ACCOUNTABILITY
4.1 Q. C. Manager
5.0 PROCEDURE
5.1 PRECAUTIONS
5.1.1 Always use freshly prepared culture slant for initial suspension which should not be more than 48 hours old.
5.1.2 Always use properly cleaned and sterilized glass containers.
5.1.3 Vortex each dilution before transferring the suspension to next tube of series.
5.1.4 Suspension of culture should be homogenous.
5.1.5 Check calibration status of colony counter before use.
5.1.6 Media should not have the temperature more than 45 oC prior adding to the plate containing dilution.
5.1.7 It is extremely important to make each serial transfer immediately after vortex.
5.2 MATERIALS REQUIRED
5.2.1 Media, Dilution tubes containing sterilized 09 ml normal saline, Micropipette, Sterilized Pipette 10 ml, sterilized microtips (100-1000µl), Inoculation Loop, Sterilized Petri plates, Sterilized Normal saline, Freshly prepared slant not more than 48hrs old.
5.3 EQUIPMENTS REQUIRED
5.3.1 Vortex Mixer, Colony counter, BOD Incubator, Refrigerator.
5.4 PROCEDURE
5.4.1 Prepare the media.
5.4.2 Sterilize the media at 121oC for 20 minutes in autoclave as per SOP.
5.4.3 Take a freshly prepared culture slant which should not be more than 48 hour old.
5.4.4 Add 10 ml of sterile normal saline to the tube and allow to stand for 10 minutes.
5.4.5 Homogenize the tube by gentle shaking to make a homogenous suspension.
5.4.6 Vortex the tube containing initial suspension for not less than one minute.
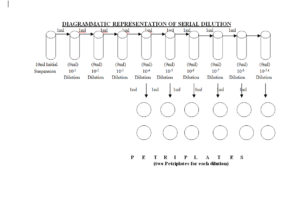
5.4.7 Transfer 1ml aliquot from initial suspension to dilution tube containing 9 ml of sterile normal saline and mark as 10-1 and vortex the dilution tubes for at least 10 seconds (1:10 dilution).
5.4.8 Transfer 1ml from 10-1 to second dilution tube containing 9 ml of sterile normal saline & mark as 10-2.
5.4.9 Repeat the step 5.4.8 eight times to make a dilution series up to 10-5 to -8( 1ml from second to third, third to fourth, fourth to fifth so on respectively and mark them as 10-3 , 10-4 , 10-5 …….respectively).
5.4.10Vortex each tube before transferring the dilution to next tube for 10 seconds.
5.4.11Perform the serial dilutions in the same manner ranging from 10-1 to 10-5 to ,-8.
5.4.12Pipette 1 ml from 10-4 tube and add to two sterilized Petri plate. Do this for dilutions up to dilution 10-5 to -8 and mark the Petri plates as per dilution.
5.4.13Pour approximately 20 ml of sterilized melted Soyabean Casein Digest Agar cooled to 45 oC to 50 oC into Petri plates containing respective dilution for bacterial cultures, Sabouraud Dextrose Agar to all the yeast or molds cultures and Columbia Agar for Clostridium sporogeneses into the plates.
5.4.14 Swirl to assure adequate mixing and allow the agar to solidify.
5.4.15 Invert the plate and Incubate the plates at 32.5 ± 2.5°C for 48 hrs for bacterial cultures and 22.5± 2.5C for 3 to 5 days for fungi & for Clostridium sporogeneses in anaerobic jar at 32.5 ± 2.5 ° C for 72 hrs.
5.4.16 Do not discard the dilutions after plating the cultures. Preserve the all dilutions in refrigerator at temperature 2-8°C.
5.4.17 After incubation count the plates and calculate the population by multiplying the count with
dilution factor.
5.4.18 Select the previous dilution of which is having cells between 10 – 100 Cfu/ml.
5.4.19Transfer 1ml from this dilution to the bottle containing 100ml sterile normal saline.
5.4.20 Store the culture suspension for 01 to 10 Days at 2-8°C.
5.4.21 Frequency of culture suspension preparation of bacterial and fungal cultures is once in 10 days.
5.4.22 Label the culture suspension with following details: –
Specimen label
| Name of organism:
ATCC/MTCC No.: No. of cfu/ml: Date of preparation: Valid up to: Prepared By : |
5.5 FREQUENCY
5.5.1 Weekly
6.0 ABBREVIATIONS
| Sr. No. | Abbreviation used | Full form of abbreviation used |
| 1.0 | SOP | Standard Operating Procedure |
| 2.0 | IPA | Iso Propyl Alcohol |
| 3.0 | QA | Quality assurance |
| 4.0 | SCM | Soyabean Casein Digest Medium |
| 5.0 | IP | Indian Pharmacopeia |
| 6.0 | HLAF | Horizontal Laminar Air Flow |
| 7.0 | Cfu | Colony Forming Unit |
7.0 ATTACHMENTS (ANNEXES)
Annex -I : Test Organism Culture Dilution Preparation Report Format
8.0 REFERENCE
| Sr. No. | Reference Title |
| 01 | Indian Pharmacopeia |
Annex -I : Test Organism Culture Dilution Preparation Report Format

sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware