sop for maintenance of cultures
1.0 OBJECTIVE
To lay down a procedure for maintaining of bacterial cultures.
2.0 RESPONSIBILTY
Microbiologist / Executive.
3.0 ACCOUNTABILITY
Head – Quality Control
3.0 PROCEDURE
4.1 Bacterial cultures are very sensitive and if not sub-cultured they can change their morphological & biochemical characteristics.
Sub-culturing or periodic transfer is a very delicate technique which has to be carried out by avoiding chances of contamination.
4.3 Prepare the media as mentioned in SOP
4.2 Prepare slants in clean 18 mm diameter rimless test tubes and pre-incubate for 48 hrs at 30-35C to check any contamination.
4.3 Boil the media till get completely dissolve. Cool to about 42 – 45°C and adjust the required pH . Fill each test tube with
about 9 to 10ml of the medium & plug the tubes with non absorbent cotton & sterilize by autoclaving.
4.4 After autoclaving, prepare the solutions by slanting the test tubes to desirable angle.
4.5 After the medium get solidified, keep the prepared slants into the incubators (37°C for 48 hrs.)for pre incubation.
4.8 Prior to sub culturing incubation is necessary because to check for the contamination of slants
occurs in process.
4.9 After incubation, clean the exterior surface of test tube with IPA 70% solution.
4.10 Mark each test tube with the name of organism & date of subculture.
4.11 At every passage from mother culture check the Gram character and morphology of the culture. In any case the passage from mother culture shall not exceed 5.
4.12 Subculture should be done in the of Laminar flow clean air station & in between the two gas burners to avoid the chances of cross contamination.
Take a loop full of culture from previous stock cultures or mother culture which ever is applicable, and inoculate in freshly prepared sterile slant
4.13 Inoculate each organism in two slants ( prepare two slants of each organism). After inoculating ,incubate all the tubes at required
temperature(i.e. 30-35°C for bacteria and 20-25°C for fungus).
4.13 After incubation check the cultures visibly for any contamination.
4.15 Mark the Newly prepared lot of cultures tubes as WORKING CULTURES and one as STOCK
CULTURE.
4.16 After observing the growth, keep the newly prepared cultured tubes in clean double plain
polythene bags and preserved the tubes in the refrigerator at 2 – 8 °C.
4.17 Prepare new slants from the STOCK CULTURE of previous month ( at each month ,as a new passage)
and should proceed from more than four passage.
4.18 After completion of two passages from a single STOCK CULTURE, take the passage from MOTHER
CULTURE slant, proceed for the next passage , by preparing STOCK CULTURE and WORKING
CULTURE.
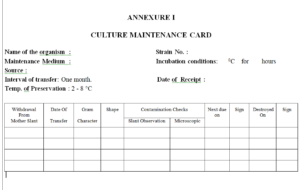
4.19 Enter the date of sub-culturing and passage number in the format as mentioned in Annexure I for sub-
culturing of micro-organism.
4.20 After every one year procure the new culture from any national recognized institution with certificate for
authenticity of the cultures.
4.21 The working cultures shall be used for preparing suspensions for positive control; for Growth Promotion
test etc.
4.22 The STOCK CULTURE shall be used only for sub culturing.
4.23 After one month when the new cultures are ready for use destroy the old cultures.
5.0 DISPOSAL OF CULTURES :
5.1 Aseptically pipette 10ml of disinfectant solution in the culture tubes.
5.2 Sterilise at 121 °C for 20 mins.
5.3 After sterilization collect the media in the double polybag, pour sufficient disinfectant solution and tie it
properly. Dispose this bag in Incinerator.
6.0 MEDIA USED FOR PREPARING SLANTS :
1. For Bacteria : Soyabean Casein digest Agar.
2. For Fungi : Sabaroud Dextrose Agar.
7.0 ABBREVIATIONS : NIL
8.0 REFERENCE
Recommendations by culture supplier.
9.0 ANNEXURE : Annexure I
sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware