sop for media preparation
1.0 OBJECTIVE
To lay down a procedure for preparing of media for microbiological tests.
2.0 RESPONSIBILTY
Microbiologist./ Q.C Executive.
3.0 ACCOUNTABILITY
Head – Quality Control .
4.0 INTRODUCTION :
4.1 Before employing any dehydrated media for analysis ensure that the growth promotion test has been
Performed for that particular batch or lot or any new media procured.
4.2 Media for Microbiological testing should be prepared as per requirements.
4.3 For preparing any culture media (Solid or Liquid) take properly cleaned glass container
of different volumes as per requirement.
4.4 Select the glass container such that the medium volume be prepared, is half of its capacity.
4.5 Follow the instructions given by the manufacturer for preparing the culture media (Name of the Mfr.
Hi-media Laboratory Pvt. Ltd.).
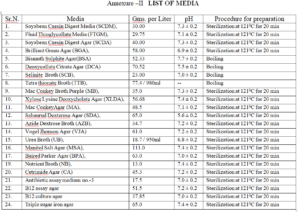
4.6 Weigh the media powder as mentioned on the label of the container and Annexure II . Reconstitute with
distilled water and mix the powder stirring with glass rod.
4.7 Warm the powder mixed distilled water, if required, to dissolve the powder completely & check the pH
of the solution with the help of pH indicator strip.
4.8 Plug the mouth of the container by non-absorbent cotton and wrap butter paper to prevent any
Contamination and label.
4.9 Boil or sterilize the media at 121°C for 20 min, as per manufacturer instruction and check the pH after
sterilization.
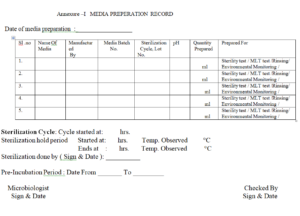
4.11 After completion of all the above procedures label each media containers with media name, media lot no.
date of preparation, and Use before date on the container . Pre-incubation must be done at 30-35 C
for at least 48 hrs before taken for use. In case if the medium is required to be use immediately, keep
appropriate negative and positive control to ensure the proper sterilization and fertility of the medium.
4.12 Record the media preparation and pre-incubation details in Media preparation record as mentioned in
Annexure I.
4.13 Prepared media can be used within 1 month period if properly sealed and stored at temperature < 25°C.
4.14 Whenever new bottle is opened write the date of opening , Date of growth promotion test and signature
on the bottle.
5.0 ABBREVIATIONS : Nil
6.0 ANNEXURES : Annexure –I
Annexure –II.
7.0 REFERENCES : In house
sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware