SOP FOR PERSONNEL QUALIFICATION PROTOCOL FOR ASEPTIC AREA
SUPERDEDES PROTOCOL No.
AREA NAME
| S. No. | Title | Page No.
|
| 1.0 | Protocol Approval | |
| 2.0 | Objective | |
| 3.0 | Scope | |
| 4.0 | Responsibility | |
| 5.0 | Reason for Qualification | |
| 6.0 | Requalification Criteria | |
| 7.0 | Training details | |
| 8.0 | Requirements for qualification | |
| 9.0 | Methodology | |
| 10.0 | Location of sampling for personnel qualification | |
| 11.0 | Acceptance criteria | |
| 12.0 | References | |
| 13.0 | Documents to be attached | |
| 14.0 | Non compliance | |
| 15.0 | Deviation from pre-defined specification, if any | |
| 16.0 | Change control, if any | |
| 17.0 | Abbreviations | |
| 18.0 | Revision history |
CONTENTS
1.0 PROTOCOL APPROVAL:
| DEPARTMENT | NAME | DESIGNATION | SIGNATURE/DATE |
| MICROBIOLOGY (QC) |
REVIEWED BY:
| DEPARTMENT | NAME | DESIGNATION | SIGNATURE/DATE |
| QUALITY ASSURANCE | |||
| ENGINEERING | |||
| PRODUCTION | |||
| QUALITY CONTROL |
APPROVED BY:
| DEPARTMENT | NAME | DESIGNATION | SIGNATURE/DATE |
| HEAD QUALITY ASSURANCE |
2.0 OBJECTIVE:
To establish methodology for Personnel Qualification in aseptic area of Microbiology Section, Production (Aseptic area)
3.0 SCOPE:
The scope is limited to evaluate the personnel entering the aseptic area, to ensure that their behavior, hygiene and operations are suitable for sterile manufacturing and to demonstrate that the qualified personnel are capable to consistently achieve desired sterility assurance for the finished product.
The purpose of this activity is to demonstrate that the personnel entering the aseptic area, who are going to perform the sterile manufacturing process, aseptic practices and manual operations during the production of Sterile Formulations, are qualified by giving training, evaluating and certifying to provide the desired Sterility Assurance in product manufacturing. This qualification is also applicable to all the other personnel who are expected to enter the aseptic area for various other activities, like Microbiologist (for routine environment monitoring), Engineering Personnel (for maintenance purpose) and QA personnel (for supervision).
4.0 RESPONSIBILITY:
The Validation Group, comprising of a representative from each of the following departments, shall be responsible for the overall compliance of this Protocol.
| DEPARTMENTS | RESPONSIBILITIES |
| Quality Control | · To conduct the qualification study as per protocol.
· To perform the microbiological monitoring. · Sampling as per protocol. · Preparation, Review and Compilation of the Personnel Qualification Protocol. · Protocol Training. |
| Quality Assurance | · Co-ordination with Quality Control, Production and Engineering to carryout Personnel Qualification Activity. |
| Production | · Review of Protocol.
· Training of personnel. · To co-ordinate and support Personnel Qualification Activity. |
| Engineering | · Review of Protocol.
· Training of personnel. · Co-ordination, Execution and technical support in Personnel Qualification activity. |
5.0 REASON FOR QUALIFICATION:
Qualification will be performing in case of:
• New Person entering in aseptic area.
6.0 REQUALIFICATION CRITERIA:
Re-qualification will be performing in case of:
• Re-qualification of all individuals shall take place once in a year ± 30 days.
• Personnel monitoring count observed out of limit.
• If person return back after recovering from any disease.
• Re-qualification reports shall be prepared annually on the basis of medical fitness; routine personnel monitoring & media fill participation of all individual.
7.0 TRAINING DETAILS:
• All the personnel involve in this activity shall be appropriately trained in their job related activities.
• Verify the training records of persons involved in the personnel qualification activity.
• All the persons who are supposed to enter the Aseptic area are required to the all training before execution of personnel qualification.
• Only trend person shall be allowed for qualification activity for entering in aseptic area.
8.0 REQUIREMENTS FOR QUALIFICATION:
8.1 All the persons who are supposed to enter the Aseptic area are required to the all training before execution of personnel qualification. Only trend person shall be allowed for qualification activity for entering in aseptic area.
8.2 A person / operator shall undergo classroom training for, Aseptic techniques, behavior and working in the aseptic area and gowning procedures. The personnel details for qualification shall be recorded in Personnel Identification.
8.3 The specific training topics for qualifying the entry into the Aseptic area for all the newly joined personnel’s are:
8.3.1 Personnel Hygiene
8.3.2 Entry & Exit of Aseptic Area of respective section.
8.3.3 Gowning procedure of Aseptic Area of respective section.
8.3.4 Cleaning & sanitation of Aseptic Area of respective section.
8.3.5 Aseptic Practices in aseptic area
8.3.6 Personnel monitoring procedure by contact plate & finger dab.
9.0 METHODOLOGY:
9.1 Trainer shall demonstrate the proper gowning procedure for entry into the aseptic area as per respective SOPs.
9.2 Executive – QC (Microbiologist) shall give basic knowledge of microbiology to all the personnel who shall be involved in aseptic process and simulation test.
9.3 After demonstration of gowning by Executive – QC, the personnel shall be asked to demonstrate the gowning procedure in the controlled area.
9.4 Those who are able to satisfy the gowning procedure shall only allow to aseptic area for personnel qualification.
9.5 Personnel shall be enter the Aseptic area as per respective SOP.
9.6 Qualified Microbiologist/IPQA shall observe whether the person is following the SOP for entry and gowning procedure and record the observations on Report.
Note: Microbiologist / IPQA shall perform the personnel monitoring during personnel qualification.
9.7 If the gowning is performed appropriately the personnel shall move to the Aseptic area, where they shall be monitored by microbiologist for bioburden
using RODAC plates as per respective SOP.
9.8 After completion of monitoring the personnel shall then exit from the aseptic area after disposing the garments.
9.9 The report of three days monitoring shall be attached with qualification report. This personnel-monitoring procedure shall be repeated for 3 continuous working days.
Note: During the initial qualification the personnel shall be allowed to enter into the core working areas during these monitoring sessions.
However, in future any newly joined personnel shall not be allowed to enter the core areas during these monitoring sessions and he shall exit the aseptic area through exit Airlock.
9.10 After completion of all qualifications, final evaluation shall be prepared and recorded in qualification report.
9.11 After satisfactory result of 3 days’ personnel monitoring, only those persons are allowed to work in Aseptic area who has participated in media
fill activity or has performed sterility activity in microbiology lab.
9.12 List of Authorized person for working in Aseptic area shall be prepared after successfully completion of personnel qualification.
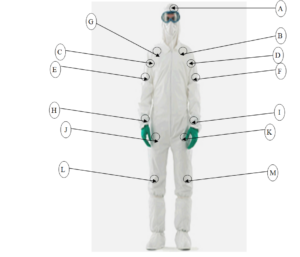
10.0 LOCATION OF SAMPLING FOR PERSONNEL QUALIFICATION: –
| SR. NO. | LOCATION CODE | LOCATION | SAMPLING METHOD |
| 1. | A | FOREHEAD | CONTACT PLATE |
| 2. | B | CHEST RIGHT | |
| 3. | C | CHEST LEFT | |
| 4. | D | RIGHT ARMPIT | |
| 5. | E | LEFT ARMPIT | |
| 6. | F | RIGHT ELBOW | |
| 7. | G | LEFT ELBOW | |
| 8. | J | RIGHT THIGH | |
| 9. | K | LEFT THIGH | |
| 10. | L | RIGHT BOOTY | |
| 11. | M | LEFT BOOTY | |
| 12. | H | RIGHT HAND FINGER DAB | FINGER DAB |
| 13. | I | LEFT HAND FINGER DAB |
10.1 LOCATION OF SAMPLING FOR PERSONNEL QUALIFICATION:-
11.0 ACCEPTANCE CRITERIA:
11.1 Person shall be considered qualified for entering into aseptic area if:
11.1.1 Person performs satisfactorily during the gowning demonstration.
11.1.2 Personnel monitoring results of 3 continuous working days meet the acceptance criteria as per SOP.
11.1.3 If person is sick or suffering from some infectious disease the person shall allowed after submission of Medical fitness certificate by registered medical practioner.
12.0 REFERENCES:
• SCHEDULE M “Good Manufacturing Practices and requirements of premises, Plant and Equipment for Pharmaceutical Product.
• FDA Guidance for Industry – Sterile Products Produced by Aseptic Processing – cGMP, September 2004
• Qualification for the personnel entering in to Aseptic Area.
13.0 DOCUMENTS TO BE ATTACHED:
• Raw data generated during testing.
• Any Other Relevant Documents.
14.0 NON COMPLIANCE:
• In case of any Non-compliance observed during PQ, inform to Head QA for necessary action.
• Document the details observed.
• The Head QA will study the impact of Noncompliance. If Noncompliance is acceptable and it does not have an impact on performance
of the Qualification, prepare final conclusion.
15.0 DEVIATION FROM PRE-DEFINED SPECIFICATION, IF ANY:
• In case of any deviation observed during PQ, inform to Head QA for necessary action.
• Document the deviation detail in observed deviation section.
• The Head QA will study the impact of deviation. If deviation is acceptable and it does not have an Impact on performance of the Qualification, prepare final conclusion.
16.0 CHANGE CONTROL, IF ANY
• If any change control is required during PQ, inform to Head QA for necessary action.
• Document the details observed.
• The Head QA will study the impact of change. If change is acceptable and it does not have an Impact on performance of the Qualification, prepare final conclusion.
17.0 ABBREVIATIONS:
QA : Quality Assurance
QC : Quality Control
No. : Number
Ltd. : Limited
ID No. : Identification Number
Ml : Milliliter
CFU : Colony forming unit
GPT : Growth promotion test
SCDA : Soybean casein digest agar
RODAC : Replicate organism detection and counting
sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware