sop for Validation protocol of steam sterilizer autoclave
TABLE OF CONTENTS
Sr. No. Title Page No.
1 PROTOCOL APPROVAL SHEET…………………………..……………..
2 OBJECTIVE…………………………………………………………………..
3 SCOPE…………………………………………………………………..……
4 RESPONSIBILITY……………………………………………………..…….
5 EQUIPMENT DESCRIPTION………………………………………………
6 REQUIREMENT……………………………………………………………..
7 LOAD PATTERN FOR VALIDATION OF AUTICLAVE………………….
8 VALIDATION METHODOLOGY FOR AUTOCLAVE………..……..……
9 SET PARAMETER…………………………………………..…………..….
10 REVALIDATION CRITERIA…………………………………………………
11 FREQUENCY OF VALIDATION……………………………………………
12 DEFINITION…………………………………………………………………..
13 REFERENCE…………………………………………………………………
14 DOCUMENTS……………………………………………………….……….
15 ABBREVIATIONS…………………………………………………………….
1.0 PROTOCOL APPROVAL
This Validation Protocol has been prepared and Reviewed and Approved by following:
DEPARTMENT NAME DESIGNATION SIGNATURE DATE
PREPARED BY
Executive – QA
CHECKED BY
Head
Quality Control
APPROVED BY
Head
Quality Assurance

2.0 OBJECTIVE:
The objective of this protocol is designed to establish sufficient data to assure that the Autoclave Supplied by
M/s. ABC equipment is suitable for sterilizing the loads. In addition, this validation protocol is intended to
assure the sterility of the items, when the equipment is operated in accordance with the established standard
operating procedure to maintain reliability and repeatability.
3.0 SCOPE:
This protocol is applicable For Vertical Autoclave, which is installed in Quality Control deptt. Microbiology section
4.0 RESPONSIBILITY:
| Department | Responsibilities |
| Microbiology | Development of Validation protocol and assembling of data into a final report.
Placing of Biological indicator & chemical indicator(strips) Collection & analysis of Biological indicator |
| Quality Control | Review of this protocol. |
| Quality Assurance | Review and Approval of protocol and of the final report. |
5.0 EQUIPMENT DESCRIPTION:
5.1 Autoclave Ist
Name of the Equipment : Steam Sterilizer (Autoclave)
Make : ABC
Equipment I D No. :
Equipment Location. : Autoclave (Microbiology Department)
5.2 Autoclave IInd
Name of the Equipment : Steam Sterilizer (Autoclave)
Make : ABC
Equipment I D No. :
Equipment Location. : Autoclave (Microbiology Department)
5.3 USE OF EQUIPMENT:
5.3.1 The Autoclave is used for decontamination of culture dilution tubes & slants, Environmental monitoring plates & tubes,
water testing plates & tubes, Raw material MLT plates & tubes, Finished product MLT plates & tubes, disinfectant testing
media tubes, used micropipette tips, positive and negative cultures plates and tubes, Environmental isolates plates & tubes
at 121° to 124°C for decontamination of the media is accomplished by heat transfer from steam to media.
6.0 REQUIREMENT:
6.1 In order to efficiently conduct validation of an Autoclave, the following requirements must be fulfilled
6.1.1 Calibrated Data logger with PT-100 sensors.
6.1.2 Biological Indicator for Pours & Liquid Load (106 spores of Bacillus stearothermophilus in
strips.
6.1.3 Culture media used for validation purpose
6.1.4 All instruments used for validation must be calibrated.
7.0 LOAD PATTERN FOR VALIDATION OF AUTOCLAVE
7.1 Empty Chamber Heat distribution studies with temperature mapping probe at different locations of the sterilizer chamber.
7.2 Loaded chamber heat Distribution & penetration studies for each sterilization load of fixed loading pattern at 121ºC.
7.3 Porous Load (Heat Penetration Studies for Sterilization of Glass wares, Accessories hand gloves etc.)
7.4 Liquid Load (Heat Penetration Studies for Sterilization of media etc.)
8.0 VALIDATION METHODOLOGY FOR AUTOCLAVE
8.1 Heat distribution studies (Empty Lode)
8.1.1 Objective:
8.1.1.1 Objective of this test is to ensure that, the steam sterilizer is capable of attaining a temperature of 1210C during the
sterilization hold period with steam pressure of 1.1 to 1.2 kg/cm2.
8.1.1.2 Temperature spread within the range of 1210C to 1240C during sterilization cycle will demonstrate the uniform
heat distribution within the chamber.
8.1.2 Procedure
8.1.2.1 Record the set parameter for the sterilization cycle to be operated during the test for empty chamber heat distribution study.
8.1.2.2 Pass minimum 8 no. Temperature mapping probe into chamber through the port of the sterilizer. Seal the port with silicone sealant so
that steam leakage does not take place. Suspend the probes in the chamber in different position so that probes do not touch any metallic.
Record the position of the probes in a respective schematic form.
8.1.2.3 Connect the probes to a suitable data logger, which can scan and print the actual temperature observed at different locations with respect to time.
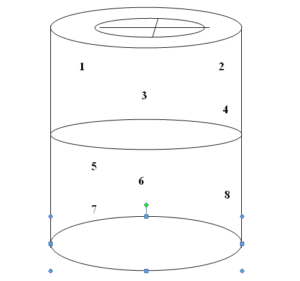
Figure 1.0 – Schematic diagram for Probe Location for Empty Cycle (1-8 are the locations for probe)
8.1.2.4 Operate the autoclave as per SOP and also start the data logger to record actual temperature within the sterilization chamber with respect to time.
8.1.2.5 When the sterilization cycle completes, Download the data-analysis and printing. Record the temperatures observed at different locations.
8.1.2.6 If the empty chamber heat distribution study is acceptable perform three consecutive runs to demonstrate cycle and sterilizer reproducibility.
8.1.3 Acceptance Criteria
8.1.3.1 Throughout the well time all temperature measured in the chamber do not fluctuate by more than 10C
8.1.3.2 The minimum accumulated F0 value should be more than biological F0 value for the Biological indicator strips exposed to the bio-challenge studies.
8.2 Loaded Chamber Heat Distribution & Penetration Studies Along with chemical & Biological Indicator (Porous Load & Liquid Load)
8.2.1 Objective
8.2.1.1 Objective of this test is to ensure that, the steam is sufficiently penetrating into the innermost portions of the load subjected for sterilization
to achieve desired Fo Value during the complete sterilization hold period.
8.2.1.2 If the minimum Fo Value is not achieved throughout the cycle, load configuration or size of the load has to be reviewed and cycle to be repeated.
8.2.1.3 Temperature spread within the range of 1210C to 1240C during sterilization hold period indicate that, uniform heating process which is
achieved in the empty chamber heat distribution study is not affected by load.
8.2.1.4 There could be the possibility of lag period for attaining 1210C during heat penetration runs as the probes are placed
deep into the load. But if minimum Fo Value is achieved at each probe, there is no need to consider for lag period. But if minimum Fo Value is not achieved at each probe, then cycle time will have to be increased so as to achieve minimum Fo Value.
8.2.1.5 The location where minimum Fo Value is observed during the sterilization cycle will be considered as cold spot.
8.2.2 Procedure
8.2.2.1 Record the set parameter for the sterilization cycle to be operated during the test for loaded chamber heat penetration study in the Annexure.
8.2.2.2 Pass minimum 8 no. Temperature mapping probes into chamber through the port provided. Seal the port with silicone sealant so that steam leakage does not take place. Place the probes inside the load components, which are supported to be most difficult points for steam penetration, also place biological indicator along with each temperature mapping probe during the last cycle of media discard. Record the position of the probes and biological indicators in a representative schematic form.
8.2.2.3 Connect the probes to a suitable data logger, which can scan and print the actual temperature with respect to time.

Figure 2.0 – Schematic diagram for Probe Location along with Biological Indicator media sterilization loaded cycle
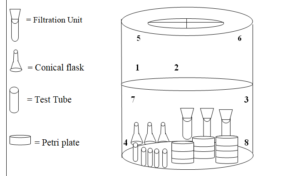
Figure 3.0 – Schematic Location of glassware at the time of sterilization
8.2.2.4 Operate the steam Sterilizer as per SOP and also start the data logger to record the actual temperatures within the sterilization
chamber with respect to time.
8.2.2.5 When the sterilization cycle completes, Record the temperatures observed at different locations. Aseptically collect the
exposed biological indicators and send the indicators to microbiology lab for further incubation and observed the results.
8.2.2.6 If the load penetration study is acceptable perform three consecutive runs to demonstrate cycle and sterilizer reproducibility.
Compile the data generated during the qualification test for complete evaluation of the system.
8.2.3 Acceptance Criteria
8.2.3.1 Throughout the dwell time all temperature measured in the chamber do not fluctuate by more than 10C.
8.2.3.2 The calculated minimum F0 value should be more than biological F0 value for the Biological indicator strips exposed to the bio-challenge studies.
8.2.3.3 No growth should be observed for exposed sterilized indicator & Negative Control (unexposed indicator) in the SCDM.
8.2.3.4 Growth should be observed in the Positive control tube that was not exposed to the Sterilization cycle.
8.3 Bio-challenge studies
8.3.1 Objective
8.3.1.1 The steam sterilization process, when challenged with Bacillus stearothermophillus biological indicator spore strip/ampoule
spore population of NLT 105 spores strip, should reduce bacterial load by mean of Sterility Assurance Level (SAL) 10-5 Biological
Indicators should be used as per the load.
8.3.1.2 For Porous & Liquid Load at 121ºC – Biological Indicator for Pours & Liquid Load (105 spores of Bacillus stearothermophillus should be used.
8.3.1.3 On incubation of the loaded biological indicator, if growth is observed, then the sterilization cycle parameters are to be reviewed.
8.3.2 Procedure
8.3.2.1 Collect the exposed indicator (during the loaded chamber heat distribution & heat penetration studies) and then send to
microbiology lab for incubation (Incubate the Geobacillus stearothermophillus indicator at 550C to 600C for 7 days).
8.3.2.2 Keep one indicator as a negative control provided by the Mfg of biological indicator as well as one indicator as a
positive control (unexposed biological indicator).
8.3.2.3 Observe any growth for sterility of media & Glassware sterilization cycle. Record the observations on daily
basis in the Annexure. Compile the data generated during the qualification test for complete evaluation of the system.
8.4 Estimation of F0 Value
8.4.1 Objective
8.4.1.1 The calculated F0 value should not be less than the biological F0 value at all temperature mapping locations during the sterilization hold period.
8.4.2 Procedure
8.4.2.1 Record the temperature at all temperature mapping probes during the sterilization hold period in the Annexure.
8.4.2.2 Calculate the F0 value at each temperature mapping probe by using the equation as below.
8.4.2.3 Record the F0 value (Results) in the Annexure.
8.4.2.4 Compile the data generated during the qualification test for complete evaluation of the system.
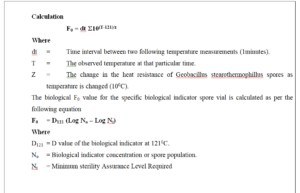
9.0 SET PARAMETER:
| Load description | Sterilization Temperature | No. of cycle | Sterilization Hold time
(Minute) |
| Heat Distribution Empty cycle | 121oC | 1 | 20 |
| Porous Load-
Minimum Load: Sterilization of Glassware (25 Nos Petri plates, 5 Nos sampling bottles), Accessories (5 Nos spatulas, 5 Nos forceps, 5 pairs Hand gloves, 4 Nos Measuring Cylinder, 4 Nos Filtration Assembly. |
121oC | 1 | 20 |
| Maximum Load: Sterilization of Glassware (100 Nos Petri plates, 8 Nos sampling bottles), Accessories (8 Nos spatulas, 8 Nos forceps, 10 pairs Hand gloves, 8 Nos Measuring Cylinder, 6 Nos Filtration Assembly.
|
121oC | 1 | 20 |
| Liquid Load
Minimum Load: Sterilization of Media (2x 100 ml SCDM, 200 ml SCDA, 100 ml SDA 100 ml CA, 100 ml MSA, 100 ml MCA, 2X100 ml MCB, 2x90ml Buffered Sodium chloride Peptone).
|
121oC | 1 | 15 |
| Maximum Load: Sterilization of Media
(10 x100 ml SCDM, 3x400ml SCDA, 10 x 90 ml Buffered Sodium chloride Peptone, 500 ml SDA, 300 ml CA, 300 ml MCA, 300 ml MSA, 10 x100 ml MCB).
|
121oC | 1 | 15 |
10.0 REVALIDATION CRITERIA:
10.1 Any major modification to any part / process of the existing autoclave which must be documented through a change control system.
10.2 Adjustments made in the equipment, as a rectification measure to counter the non compliance of the Results.
11.0 FREQUENCY OF VALIDATION:
11.1 The frequency of Periodic Validation for is yearly basis (±15 Days) for existing systems.
12.0 DEFINITIONS:
12.1 STERILE: In strict sense a specimen would be deemed sterile only when there is complete absence of viable microorganisms from it.
12.2 STERILIZATION: A process, by which all viable microorganisms are removed or destroyed, based on a probability function.
12.3 BIO BURDEN: The number of viable microorganisms in or an object or preparation entering a sterilization step
(usually expressed as colony forming unit per unit volume).
12.4 D VALUE: The D value is the time (in minutes) required to reduce the microbial population by 90% or 1 log cycle (i.e. to a surviving fraction of 1/10).
12.5 Z VALUE: The Z Value is the temp. (in ºC) required for one-log reduction.
12.6 F0 VALUE: The F0 Value is the equivalent time for which a monitored article is exposed to the temperature of 1210 C.
12.7 BIOLOGICAL INDICATORS (BIs): BIs are live spore forms of microorganisms known to be the most resistant living organisms to the
lethal effects of the particular sterilization process. For steam sterilization, the most resistant microorganism is Bacillus stearothermophilus.
12.8 STERILITY ASSURANCE LEVEL (SAL): A term related to the probability of finding a nonsterile unit following a sterilization step.
It is usually expressed in the negative power of 10 (i.e. one in one million = 10 –5).
12.9 PULSE: A sub cycle at the start of Sterilization Cycle in which Vacuum and Steam are alternatively supplied to a steam
sterilizer chamber, for the purpose of removal of air from the chamber.
13.0 REFERENCES:
Schedule – M – “Good Manufacturing Practices and Requirements of Premises, Plant and Equipment for Pharmaceutical Products.”
14.0 DOCUMENTS
14.1 Any Change in the protocol and activity shall be documented and supported with change control.
14.1.1 Protocol No. along with Annexure
14.1.2 The report shall be prepared by Microbiologist, Reviewed by Head-QC and approved by Head-QA. The report shell have following as its Annexure
14.1.3 Calibration certificates.
14.1.4 Certificates of biological indicator.
14.1.5 Chemical indicator.
14.1.6 Any other relevant documents (if any).
15.0 ABBREVIATIONS
15.1 MHS : Moist Heat Sterilizer
15.2 NA : Not Applicable
15.3 RTD : Resistant Temperature Device
15.4 QA : Quality Assurance
15.5 QC : Quality Control
15.6 SAL : Sterility Assurance Level
15.7 °C : Degree Centigrade
15.8 SOP : Standard Operating Procedure
15.9 NMT : Not More Than
15.10 VMP : Validation Master Plan
15.11 BI : Biological Indicators
15.12 A.R. : Analytical Report
15.13 BP : British Pharmacopoeia
15.14 WHO : World Health Organization
15.15 cGMP : Current Good Manufacturing Practice
16.0 DOCUMENT CHANGE HISTORY
First time prepare.
sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware