STP for sterility testing of sterile gloves
1 OBJECTIVE
To describe the procedure for the sterility testing of sterile gloves.
The purpose of this exercise is to ensure that the gloves are free from any microbial contamination.
2 SCOPE
This procedure is applicable for the sterility testing of sterile gloves
3 RESPONSIBILITY
Microbiologist
4 ACCOUNTABILITY
Manager -QC
5 ABBREVIATIONS
QA : Quality Assurance
QC : Quality Control
HLAF : Horizontal Laminar Air Flow
IPA : Isopropyl Alcohol
WFI : Water for injection
SCDM : Soyabean Casein Digest Media
FTGM : Fluid Thioglycollate Media
NO. : Number
RH : Relative humidity
UV : Ultra-violet
6 PRECAUTIONS
6.1 All the apparatus and material used for sterility should be sterile.
6.2 All accessories like scissor and forceps used during test should be sterilized time to time with flame.
6.3 Media should be clear and free from particles.
6.4 Use proper sterile dress during test.
6.5 No other person except microbiologist is allowed to go inside the sterility room.
6.6 Start the work after proper environmental conditions are maintained for sterile area.
6.7 Cut the sterile gloves with the help of scissor near flame.
6.8 Always check sterilization status for validity of all the materials before using for sterility test.
7 MATERIAL REQUIRED
Glass beaker, Sterile forceps, Sterile scissor, Soybean casein digest media, Fluid thioglycollate media Peptone water, Sterile garments,
Sterile gloves, Isopropyl Alcohol 70% (Filtered), Disinfectant solution, Tray, SS table, Sterilization indicator strips, glass bottles,
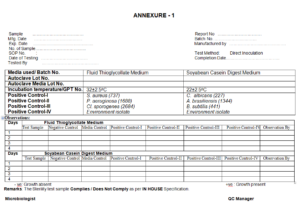
Aluminium foil, Non absorbent cotton, sterility test tube, glass bottles, Standard Positive Cultures- Bacillus subtilis
(aerobic bacteria), S. aureus (aerobic bacteria), P. aeruginosa (aerobic bacteria), Cl. sporogenes (anaerobic bacteria),
Candida albicans (fungus ), Aspergillus brasiliensis (fungus), Environmental Isolate.
8 EQUIPMENT REQUIRED
Double door autoclave, Laminar Air Flow, Bunsen burner, BOD incubator maintained at 22.5˚ + 2.5˚C for fungal growth,
BOD incubator maintained at 32.5 ˚+ 2.5˚C for bacterial growth.
9 PROCEDURE
9.1 Pre Preparation
9.1.1 Maintain the sterile condition of area by fumigation before performing the test as per
SOP if required.
9.1.2 Maintain the environment condition of the area before start of the work.
9.1.3 Check the relative humidity and temperature of the sterility area prior to the sterility testing as per
SOP. In sterility room % RH should be within 55+ 5% & temperature should
be 27˚C + 2˚C.
9.1.4 Sterile media at 121˚C for 20 minutes and accessories at 121˚C for 30 minutes before start of the
work as per SOP
9.1.5 Place sterile garments in garment cubicle provided in sterility area.
9.1.6 Place sample in Dynamic pass box after cleaned with filtered 70% IPA
solution using lint free cloth.
9.1.7 Switch on UV light and blower of HLAF 30 minutes before start the work.
9.2 Media preparation –
9.2.1 Soybean casein digest media- Weigh dehydrated media as per the requirement and dissolve the
weighed qty. in sufficient quantity of WFI to get required concentration as per manufacturer
(written on the container) of media.
9.2.2 Fluid Thioglycollate media- Weigh dehydrated media as per the requirement and dissolve the
weighed qty. in sufficient quantity of WFI to get required concentration as per manufacturer
(written on the container) of media.
9.2.4 Transfer 500ml of each media, SCDM & FTGM in bottles separately for test. Close the lids of
bottles and cover with aluminium foil. For media and positive control transfer 100 ml of each
media, SCDM & FTGM in test tubes separately. Plug the tubes with non absorbent cotton and
cover with Aluminum foil.
9.3 Sterilization of media and utensils-
Place the media bottles and tubes in autoclave and sterilize at 121˚C for 20 minutes and
other accessories like scissor, forcep for 30 minutes. Also place steam sterilization chemical
indicator inside the autoclave for sterilization verification for each cycle.
9.4 Arrangement of sample-
Take two pair of gloves of the lot to be tested. Keep it in a tray mopped with filtered 70% IPA.
9.5 Test procedure –
9.5.1 Cleaning of Sterility room-Enter into the sterile area as per SOP for Entry/Exit to Micro Lab
Clean the sterility room as per SOP for Cleaning of Micro Lab
9.5.2 Before entering in sterile area ensure that the samples are kept in the dynamic pass box.
9.5.3 After 20 minutes enter into the sterile area as per SOP for Entry/Exit to Micro Lab
9.5.4 Take out the contact plates from the dynamic pass box and carry out finger dab as per SOP for
Environmental monitoring in Third change room.
9.5.5 Collect samples from dynamic pass box and take them into sterility room & place them on SS table and
ensure that the UV light of HLAF is turned “ON” before 30 minutes.
9.5.6 Collect the sterility media bottles and tubes, equipments required for sterility test & take to the
Sterility room. Place them of SS table.
9.5.7 Switch “OFF” UV light of HLAF.
9.5.8 Mop the HLAF bench with filtered 70% IPA by using sterile lint free mops.
9.5.9 Spray filtered 70% IPA on the media bottles, tubes and on hands. Place media bottles and tubes
on HLAF bench.
9.5.10 Mark the media bottles with details like Product Name, lot No and starting date before
Performing the test.
9.5.11 Take out the gloves aseptically. Cut them with the help of sterile scissor into pieces near flame.
9.5.12 Add them into media bottles (Fluid Thioglycollate media and Soybean casein digest media)
9.5.12 Close the lids tightly.
9.5.13 Take one tube of each media, Fluid Thioglycollate media and Soybean casein digest media,
Mark them as media control.
9.5.14 Perform the test for positive control in MLT room. Bring the media tube for positive control to
MLT room.
9.5.15 Make a positive control tube by transferring 1ml suspension of working dilution of standard
Culture to the SCDM and FTGM as per table 1 of SOP for Growth Promotion test
9.5.25 Incubate FTGM bottles of test, tubes of media control for 14 days and tubes of Positive Control
for 5 days at 32.5˚C + 2.5˚C to detect the bacterial growth & incubate SCDM bottles of test,
tubes of media control for 14 days and tubes of positive control for 5 days at 22.5˚C + 2.5˚C for
the detection of fungi.
9.5.26 Observe each test bottle daily and record the results as positive or negative growth. The
Medium should be clear and transparent .The sample passes the sterility test and test is valid
only if positive control shows growth with turbidity and negative remains clear without growth.
10 ENCLOSURES
ANNEXURE -1 STERILITY OBSERVATION SHEET OF STERILE GLOVES
11 REFERENCE
In House
sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware