sop for calibration and validation of micro autoclave
1.0 OBJECTIVE
1.1 To describe a procedure for calibration and validation of laboratory autoclave.
2.0 SCOPE
2.1 This procedure is applicable for calibration and validation of laboratory autoclave in Microbiology Laboratory
3.0 RESPONSIBILITY
3.1 Officer Microbiologist
3.2 Executive Microbiologist
4.0 ACCOUNTABILITY
4.1 Head – QC
5.0 PROCEDURE
5.1 Calibration
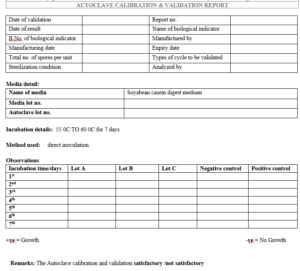
5.1.1 Put biological indicator spore strip (Bacillus stearothermophilus) inside the autoclave
with sample load at various position.
5.1.2 Operate the autoclave for 15 minute at 15 psi and 1210C temperature.
5.1.3 After sterilization cycle is over Switch ‘OFF’ the main supply, after the specified period and open the steam release valve & wait for cool the autoclave, transfer aseptically the spore strips one by one to 100-100 ml Sterile Soyabean Casein Digest Medium containing tubes and incubate at 50ºC-55ºC for 7 days.
5.1.4 After incubation period, if no growth is observed, autoclave shall be considered calibrated.
5.1. 5 Calibration Frequency: Once in 6 month or after major maintenance.
5.2 Validation
5.2.1 Temperature calibration (Heat distribution in empty chamber autoclave)
5.2.1.1 Place thermocouples at different positions in empty chamber of autoclave.
5.2.1.2 Close the autoclave and operate the cycle. After achieving the 121°C temperature record the temperature of
the probes at five minutes intervals for 15 minutes.
5.2.1.3 Carryout the study of uniform heat distribution in the empty autoclave chamber for three consecutive runs.
5.2.2 Temperature calibration (Slow to Heat point distribution)
5.2.2.1 Place thermocouples at different positions in half load of autoclave.
5.2.2.2 Close the autoclave and operate the cycle. After achieving the 121°C temperature, record the temperature of the probes at five minutes intervals for 15 minutes.
5.2.2.3 Carryout the study of slow to heat point determination in the half load of autoclave for three consecutive runs.
5.3 Final Validation
5.3.1 Select five thermocouples showing minimum temperature out of ten thermocouples used in study of slow to heat point determination.
5.3.2 Place five thermocouples at the different cold points in full load and sixth thermocouple at the drain point.
5.3.3 Place biological spore’s strips (Bacillus stearothermophilus) at all five cold points and one at the drain point.
5.3.4 Close the autoclave and operate the cycle. After achieving the 121°C temperature, record the temperature of the probes at five minutes intervals for 15 minutes.
5.3.5 Carryout the study of slow to heat point determination in the full load of autoclave for three consecutive runs.
5.3.6 After autoclaving is over take out the spore strips.
5.3.7 Incubate the autoclaved spore strips at 50°C-55°C and one un-autoclaved strips as positive control for 7 days.
5.3.8 Above said calibration shall be performed for by authorized out source calibration party once a year and whenever a major maintenance is done.
5.3.9 Frequency: Once in a year.
6.0 REFERENCE
In House
7.0 FORMATS
Annexure : Autoclave Calibration & Validation Report
8.0 ABBREVIATIONS
QCM : Quality Control Microbiology
SOP : Standard Operating Procedure
QA : Quality Assurance
QC : Quality Control
0C : Degree centigrade
9.0 REVISION HISTORY
| Revision | Reason for Revision | Effective Date |
| 00 | NEW SOP |
Annexure
sop for calibration and validation of micro autoclave
sop for Sterility failure investigation
cleaning and operation of discard autoclave
sop for operation of fogger machine
sop for Biological assay of lactic acid bacillus
sop for preparation of culture inoculum
STP for sterility testing of sterile gloves
sop for Operation and calibration of active air sampler
sop for transfer of material for testing and sampling in sterile area
entry & exit procedure in microbiology laboratory
Growth Promotion Test In Microbiology Laboratory
Operation of B.O.D in Microbiology Laboratory
Operation of Horizontal Laminar Air Flow in the microbiology laboratory
Operation and cleaning of Pass Box.
Operation and cleaning of air sampler
Cleaning and Sterilization of Glassware
Analysis of water for microbial load in microbiology lab
Operation and temperature monitoring of Refrigerator
Fumigation of Microbiology Laboratory.
Entry & Exit procedure In Sterility Area
SOP for Microbial analysis of Raw Material Finished Products
SOP for Operation & Calibration of pH meter in Micro Department
SOP for Operation & Calibration of pH meter in Micro Department
SOP Operation and calibration of Hot Air Oven In Microbiology
SOP for operation cleaning & calibration of Digital colony counter
SOP for Operation And Cleaning of Microscope
sop for Media Preparation and Consumption
sop for Receipt Storage and Usage of Culture Media
sop for Cleaning Sanitization And Disinfection In Microbiology
sop for Environmental monitoring of all the Classified area
sop for Handling and Sub culturing of Microbial cultures
sop for Media Growth Promotion Test and various Microbiological test
sop for BOD incubator operation and cleaning
sop sampling of water for microbiological analysis
sop for Disinfectant Efficacy Test
sop for for cleaning and operation of vortex mixture
sop for Temperature & Relative Humidity Monitoring
sop for Operation and Calibration of Heating Block
sop for Sterility Testing of Microbiology
sop for Disposal of Culture Media
sop for Drain point of Microbiology
sop for entry & exit procedure In Microbial limit test and Biosafety
sop for Gram Staining of Bacteria in Microbiology Laboratory
sop for Monitoring of Compressed Air/gases for microbiological analysis
sop for BET (Bacterial Endotoxin) test in Microbiology
sop for receipt storage and Determining the population of Biological indicators
sop for qualification of analyst microbiologist
sop for Bioburden test of Packing materials in Microbiology Laboratory
sop for microbiological assay of erythromycin antibiotic
sop for liquid particle counter
sop for operation and calibration of digital zone reader
sop for monitoring of ultraviolet efficiency LAF and pass box
microbiological assay of cyanocobalamin or vitamin B12
gowning procedure for microbiological testing area
swab testing of various surfaces for bioburden determination
sop for endotoxin challenge test
Hold time study protocol for sterilized media
sop for personnel Qualification protocol for aseptic area
sop for sampling and testing of drain water
Sop for Operation of Airborne Particle Counter
sop for Validation protocol of steam sterilizer autoclave
sop for pathogen detection from drain point
Sop for Analysis of Raw water Purified water water for injection and pure steam water
sop for preservatives efficacy test
sop for collection and preservation of in house isolated microorganisms
sop for Operation Calibration and Maintenance of Micropipette
sop for microbiological testing of water
sop for depyrogenation of apparatus
sop for fertility test growth promotion test of media
sop for Operation and cleaning of moist heat sterilizer
sop for monitoring by active air sampler
sop for swab sampling and testing for clean rooms in production area
sop for monitoring in microbiology laboratory
sop for Fumigation of aseptic area and microbiology lab
sop for monitoring of personnel in aseptic area
sop for maintenance of cultures
sop for Operation and cleaning of laminar bench
sop for monitoring of pure steam
sop for entry and exit procedure to m.l.t and b.e.t room
sop for storage of and use of media
sop for disposal of microbiological media and cleaning of microbiological glassware