sop for Analysis of Effluent Treatment Plant Water
1.0 Objective
To lay down a procedure for Analysis of Water of Effluent Treatment Plant Water.
2.0 Scope
This Standard Operating Procedure is applicable for Analysis of Effluent Treatment Plant Water to be followed at formulation plants
3.0 Responsibility
3.1 Officer / Executive Engineering shall be responsible for the execution of this procedure.
3.2 Engineering Manager shall be responsible for the implementation of this SOP.
3.3 Head QA/designee shall be responsible for the compliance of this SOP.
4.0 Abbreviations and Definitions
SOP : Standard Operating Procedure etc
QC : Quality control
QA : Quality Assurance
No : Number
KCl : Potassium Chloride
HCl : Hydrochloric acid
Mv : Millivolt
Hrs : Hours
oC : Degree centigrade
ppt : Precipitate
NMT : Not more than
5.0 Procedure
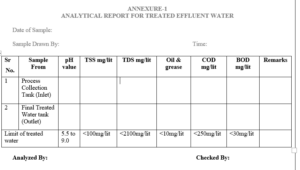
5.1 Executive – Engineering shall collect the sample of treated water on daily basis in a clean bottle.
5.2 Specification and Method of Analysis for Treated Effluent Water.
| Specification | frequency | Limits/Acceptance criteria |
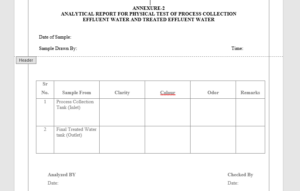
| Description | Weekly | Colorless, slight turbid liquid preferably free from unpleasant odor. |
| pH | Daily | 5.5 to 9.0 |
| Total suspended solid | Weekly | NMT 100 mg/liter |
| Total dissolved solid | Weekly | NMT 2100 mg/liter |
| C.O.D(Chemical oxygen demand) | Weekly | NMT 250 mg/liter |
| B.O.D(Biological oxygen demand) | Weekly | NMT 30mg/liter |
| Oil and Grease | Weekly | Not to exceed 10 mg/liter |
| Mixed Liquor Suspended Solids (MLSS) | Weekly | 2500 to 3000 mg/lit as per100ml Sample. |
| Mixed Liquor Volatile Suspended Solids | Weekly | 2000 to 2500 mg / lit |
| Sludge Setting Volume (SVM) | Weekly | |
| Dissolved oxygen (DO) | Weekly |
5.3 Method of Analysis:
5.3.1 Description: Take 10ml of treated effluent water in a clean and dry test tube. Check the clarity, colour and odour of treated effluent water and record the result in the Annexure-4.
5.3.2 pH: Take about 50ml of treated effluent water in a clean and dry beaker and measure the pH by a calibrated pH meter.
5.3.2.1 Acceptance criteria between5.5 -9.0.
5.3.3 Total Suspended Solids (TSS)
5.3.3.1 Aim: To determine the total suspended solids of given sample.
5.3.3.2 Apparatus and Equipment:
5.3.3.2.1 Gooch crucible / filter paper
5.3.3.2.2 Laboratory oven
5.3.3.2.3 Vacuum pump.
5.3.3.2.4 Digital analytical balance.
5.3.3.3 Procedure:
5.3.3.3.1 Weigh clean, dry Gooch crucible after drying it at 105oC for 2 hours and cool it to room temperature.(‘A ‘gm).
5.3.3.3.2 Take 50ml of treated effluent water in calibrated measuring cylinder.
5.3.3.3.3 Filter the treated effluent water through the Gooch crucible or filter paper and then wash the crucible with 10ml purified water and dry the Gooch crucible at 105oC till consecutive weights are constant. Cool Gooch crucible in a desiccators and weigh (‘B’ gm).
5.3.3.3.4 Calculation:
Total suspended solid = ( B – A) x 10,00,000/ml of sample taken
Where B = Final Wt in gm
A = Initial Wt in gm
V = ml of sample taken.
Acceptance criteria: NMT 100 mg/liter.
5.3.4 Chemical Oxygen Demand (Cod)
5.3.4.1 Aim: To determine chemical oxygen demand of the sample, boil mixture of potassium dichromate and sulphuric acid destroys most types of organic matter. A sample is refluxed with known amount of potassium dichromate and sulphuric acid the excess of potassium dichromate is titrated with ferrous ammonium sulphate. The amount of oxidisable organic matter, measured as oxygen equivalent is proportional to the potassium dichromate consumed.
5.3.4.2 Apparatus and Equipments:
• Condenser
• Flat round bottom flask
• Heating mental / Water Bath
• Standard potassium dichromate solution (0.25 M)
• Ferrous Ammonium sulphate solution(0.1 M)
• Con. Sulphuric acid
• Ferroin indicator.
• Mercuric sulphate
5.3.4.3 Potassium Dichromate solution(0.25M)
5.3.4.3.1 Dissolve 12.259 gm K2Cr2O7- analytical reagent grade previously dried at 103oC for 2 hrs. and dilute it to 1000ml with purified water.
5.3.4.4 Ferrous Ammonium Sulphate solution(0.1 M).
5.3.4.4.1 Weight accurately 39.2 gm of ferrous ammonium sulphate and dissolve it in200ml of purified water, add slowly 20 ml of Con. H2SO4 and cool, then dilute it to 1000ml.
5.3.4.4.2 For standardization, dilute 10.0 ml of K2Cr2O7 to about 100 ml, add 30 ml of Conc. H2SO4, and titrate with ferrous ammonium sulphate using ferroin as an indicator.
5.3.4.5 Ferroin Indicator: Dilute 1.485 gm of 1,10-phenonthroline and 0.695gm of ferrous sulphate (FeSO4.7H2O) in purified water to make 100ml of solution.
5.3.4.6 Sulphuric acid: H2SO4 .Concentrated ( specific gravity 1.84)
5.3.4.7 Mercuric Sulphate:HgSO4, solid
5.3.4.8 Silver Sulphate:AgSO4, solid
5.3.4.9 Procedure:
5.3.4.9.1 Place 0.4 gm mercuric sulphate (HgSO4) in refluxing flask and disolve it in 5 ml con. H2SO4.
5.3.4.9.2 Add 20ml treated effluent water (sample) and swirl to mix.
5.3.4.9.3 Add 10 ml 0.25M standard potassium dichromate solution.
5.3.4.9.4 Carefully add 25 ml of Con. H2SO4 cool the refluxing flask in ice bath.
5.3.4.9.5 Add some glass beads and shake the mixture then attach the flask to condenser and reflux the mixture for 2 hrs.
5.3.4.9.6 Cool the flask to room temperature and then wash interior side of the condenser with 80 ml purified water.
5.3.4.9.7 Titrate the excess of potassium dichromate with pre standardized 0.1M ferrous ammonium sulphate solution using 3 to 4 drops ferroin indicator until the colour changes from blue-green to reddish-brown.
5.3.4.9.8 In the same manner reflux 20ml of purified water as a blank simultaneously with the sample.
5.3.4.9.9 Calculation: (a – b) x M x 8000 /Volume of sample (ml)
Where,
a = Volume of Fe (NH4)2(SO) 46H2O used for blank
b = Volume of Fe (NH4)2(SO) 4 6H2Oused for sample
M =Molarity of 0.1M Fe (NH4)2(SO) 46H2O
5.3.5 Dissolved oxygen (DO):
5.3.5.1 Aim: In the presence of Oxygen, Manganous sulphate reacts with the alkali (KOH or NaOH) to form a white precipitate of Manganous hydroxide, gets oxidized to a brown coloured compound. In a strong acid medium Manganic ions are reduced by iodide ions, which get converted in to iodine equivalent to the original concentration of oxygen in the sample. The iodine can be titrated against Sodium thiosulphate using starch as an indicator.
5.3.5.2 Apparatus and Equipments:
• Conical flask
• Burette
• BOD bottles of 300 ml.
• 1 ml pipette
• 250 ml measuring cylinder.
• Starch solution
• Manganese Sulphate
• Alkali iodide azide solution
• 0.025M Na2S2O3
• Potassium bi iodidate
• Concentration Sulphuric acid.
• Potassium Iodide.
5.3.5.3 Preparation of Manganous Sulphate solution
5.3.5.3.1 Weigh accurately 100 gm of Mnso4 4 H2O in 200 ml of purified water and filter
5.3.5.4 Preparation of Alkali iodide azide solution:
5.3.5.4.1 Dissolve 500g NaOH or 700g KOH and 150 g of KI in purified water to make 1 liter of solution (a).
5.3.5.4.2 Dissolve 10g of NaN2 in 40ml of distilled water (b).
5.3.5.4.3 Mix the two solutions (a) and (b)
5.3.5.5 Preparation of Potassium Iodide solution:
5.3.5.5.1 Dissolve 100 gm KOH and 50 gm KI in 200 ml boiled water.
5.3.5.6 Preparation of Standard 0.025N Sodium Thiosulphate solution:
5.3.5.6.1 Dissolve 24.82g Na2S2O3 5 H2O in boiled purified water and make up the volume to 1 liter, Add 0.4g of borax or a pallet of NaOH as a stabilizer, this is 0.1 N stock solutions. Dilute it to 4 times with boiled distilled water to prepare 0.025 N solutions (250 to 1000 ml). Keep in a brown glass Stoppered bottle.
5.3.5.7 Starch solution
5.3.5.7.1 Dissolve 1 gm of starch in 100 ml of warm (80ºC- 90ºC) purified water and add a few drops of formaldehyde solution.
5.3.5.8 Procedure:
5.3.5.8.1 Take effluent sample in 300 ml BOD bottle carefully, to avoid any kind of bubbles.
5.3.5.8.2 Remove the air bubble in the bottle after placing the stopper by wrapping.
5.3.5.8.3 Add 2 ml MnSO4 solution then add 2 ml alkali azide reagent by using separate pipettes for each solution.
5.3.5.8.4 When precipitate appear, stopper carefully to exclude air bubbles and mix by inverting the bottle for few minutes.
5.3.5.8.5 Keep the bottle aside to settle down the precipitate.
5.3.5.8.6 When precipitates settled sufficiently then add 2 ml con.H2SO4 and re-stopper the bottle and shake well to dissolve the ppt. completely.
5.3.5.8.7 Take 200ml of this sample in a conical flask and titrate the contents against the previously standardized Sodium thiosulphate solution using Starch solution as an indicator. At the end of titration the colour of solution changes from deep blue to colorless.
5.3.5.8.8 Calculation:
DO = Burette reading x 1000 x Normality of Na2S2O3 /Ml of sample take
5.3.6 Biological oxygen demand (BOD)
5.3.6.1 Aim: To measure the dissolved oxygen demand Biochemical Oxygen Demand (BOD) is the measure of the degradable organic material present in water and can be defined as the amount of oxygen required by the microorganism in stabilizing the biologically degradable organic matter under aerobic condition. The principle of the method involves measuring it for 3 days at 20oC ± 1oC.
5.3.6.2 Procedure:
5.3.6.2.1 Take 2 liters of purified water in a plastic bucket/ container and purge compressed air for about 24 hrs.
5.3.6.2.2 Add 1ml of each phosphate buffer, magnesium sulphate, calcium chloride, ferric chloride solution and 1 ml of sewage water for each one liter and mixed thoroughly.
5.3.6.2.3 Neutralize the sample to pH around 7.0 by using 1M NaOH or H2SO4.
5.3.6.2.4 Since the DO in the sample is likely to be exhausted it is necessary to prepare a suitable dilution of the sample according to the expected BOD range.
5.3.6.2.5 Prepare dilution in a measuring cylinder and mix the content thoroughly. Fill two sets of the BOD bottles.
5.3.6.2.6 Keep one set of bottles in BOD incubator maintained at 20oC for 3 days and determine the DO content in other set of bottle immediately.
5.3.6.2.7 Determine DO of the incubated sample bottles after the completion of 3 days incubation.
5.3.6.2.8 For determination of DO content all the sample shall titrated with 0.025M Na2S2O3 using the starch solution as an indicator till the colour changes from blue to colorless.
5.3.6.2.9 Calculation:
BOD = ( B1 –B2 ) – (D1-D2)
Where
B1= D.O. of dilute sample before incubation (1st day)
B2= D.O. of dilute sample after incubation (3rd day)
D1=D.O. of blank before incubation (1st day)
D2= D.O. of blank after incubation (3rd day)
F= Dilution factor
F = Normality of Sodium Thiosulphate x 8 x1000 x dilution for BOD (600ml
Quantity of sample taken for DO
Acceptance Criteria: NMT 30 mg/ liter
5.3.7 Oil and grease:
5.3.7.1 Aim: To measure the oil and grease content of given sample
• Evaporating dish
• Separating funnel
• Petroleum Ether
• Water Bath
5.3.7.2 Procedure
5.3.7.2.1 Take 250 ml of sample in 500 ml separating funnel.
5.3.7.2.2 Acidify the sample by adding 2 to 3 ml of HCl (1:1) and shake well.
5.3.7.2.3 Add 50 ml of petroleum ether in the separating funnel shake vigorously for 2 minutes.
5.3.7.2.4 Allow the ether layer to separate. Collect the aqueous layer into another separating funnel.
5.3.7.2.5 Add the 50 ml of ether into aqueous layer of the separating funnel again shake it for 2 min and collect the ether layer into the separating funnel containing previous ether layer.
5.3.7.2.6 Pass the ether layer through the Sodium sulphate bed previously moistens with ether and collect the ether layer in pre-dried and pre-weighed evaporating dish (W1).
5.3.7.2.7 Evaporate all ether extract on water bath till dryness.
5.3.7.2.8 Put evaporating dish in the oven at 105o C for complete dryness.
5.3.7.2.9 Cool the evaporating dish in a desiccator and weigh the evaporating dish (W2).
5.3.7.2.10 Calculation:
Oil And Grease=
Oil And Grease= (W2 – W1) x1000 x 1000 / Quantity of sample = mg/ltr
Acceptance Criteria: Not to exceed 10mg/ liter
5.3.8 Total dissolved solids ( TDS ) :
5.3.8.1 Aim: To determine the total dissolved solids in given sample
5.3.8.2 Apparatus and Equipment:
• Evaporating dish
• Filter paper
• Laboratory Oven
• Hot plate
• Vacuum Pump.
• Digital analytical balance
5.3.8.3 Procedure:
5.3.8.3.1 Take an evaporating dish and ignite it at 550 +- 50oC in a muffle furnace for about 1 hour, cool in desiccator and weigh. (‘A’ g).
5.3.8.3.2 Filter the sample through glass fiber filter paper applying the suction.
5.3.8.3.3 Evaporate 50 ml of this filtered sample ( or more in case the solids are less than 25 mg/l ) in the pre weighed evaporating dish on a water bath or a hot plate having temperature not more than 98oC.
5.3.8.3.4 Heat the residue at 103oC – 105oC in an oven for one hour and take the final weight after cooling in a desiccator (‘B’ gm).
5.3.8.3.5 Calculation:
Total Dissolved Solid = (B – A) x 10, 00,000 /V=mg/ltr
Where, B = Final wt in gm
A = Initial wt in gm
V = ml of sample taken
Acceptance criteria: NMT 2100 mg/Liter
5.3.9 Mixed Liquor Suspended Solids (MLSS) & Mixed Liquor Volatile Suspended Solids (MLVSS)
5.3.9.1 Aim: To measure the quantity of Mixed Liquor Suspended Solids (MLSS) & Mixed Liquor Volatile Suspended Solids (MLVSS) in mg per liter of effluent.
5.3.9.2 Apparatus and Equipment:
• Evaporating dish
• Filter paper
• Muffle furnace
• Desiccator
• Laboratory Oven.
• Gooch Crucible.
• Vacuum Pump.
• Digital analytical balance.
5.3.9.3 Procedure: Preparation of asbestos pad in gooch crucible & procedure of MLSS & MLVSS
5.3.9.3.1 Take 10g. (approx.) asbestos in glass beaker.
5.3.9.3.2 Add 200ml of distilled water & add 2ml conc. Hydrochloric Acid.
5.3.9.3.3 Then shake well on magnetic stirrer & make homogeneous slurry type solution.
5.3.9.3.4 Take one crucible & put one ordinary filter paper on hole of the crucible.
5.3.9.3.5 Add asbestos solution & make pad about 2mm to 3mm using vacuum pump.
5.3.9.3.6 Dry it at 500°C in muffle furnace for completely drying (generally 2hrs.) then cool it in desiccator & take initial weight of crucible.
5.3.9.3.7 Then put this crucible on vacuum pump & filter the sample using suitable quantity.
5.3.9.3.8 Drying at 105°C for completely drying ,then cool it in desiccator (generally drying for 2hrs in oven & then cool for 30min. in desiccator), then check final weight for MLSS on analytical balance.
5.3.9.3.9 Then put this crucible at 500°C in muffle furnace for 2hrs.then cool in desiccator for 30min.& check weight for MLVSS.
5.3.9.3.10
- Calculations :
Mixed Liquor Suspended Solids (M.L.S.S.)
= (B – A) x 106 = (in mg/ltr)
ml of sample taken
Mixed liquor volatile suspended solids MLVSS)in mg/lt)
= (B – C) x 106
ml of sample taken
Where
A = Initial weight of the gooch crucible.
B = weight of the gooch crucible after drying at 105°C.
C = weight of the gooch crucible after drying at 500°C.
5.3.10 Sludge settling volume ( SVM ):
5.3.10.1 Aim: To describe the procedure of aeration tank sample.
5.3.10.2 Procedure:
5.3.10.2.1 Take 1000 ml aeration tank sample in glass cylinder.
5.3.10.2.2 Keep it a side for settling.
5.3.10.2.3 After 30minute check the settling volume for aeration tank sample, generally 1/3 of the volume taken is satisfactory.
6.0 Forms and Records
6.1 Analytical Report For Treated Effluent Water – Annexure-1
6.2 Analytical Report For Color and Odor of Treated Effluent Water – Annexure-2
6.3 pH Report For Inlet, Equalization Process Tank, Flax Mixer and Outlet (Treated Effluent Water) – Annexure-3
6.4 Mixed Liquor Suspended Solids (MLSS) & Mixed Liquor Volatile Suspended Solids (MLVSS) in mg per liter of effluent – Annexure-4
7.0 Distribution
7.1 Master copy – Documentation Cell (Quality Assurance)
7.2 Controlled copies – Engineering Department


sop for Execution of Preventive Maintenance
Preparation of Sodium Hydroxide Solution
sop for water cooled chiller operation
cleaning and preventive maintenance of LAF and RLAF
sop for replacement of water system filters
cleaning and preventive maintenance of insectocutor
sop for operation of preventive maintenance
sop for material receiving and consumption record
sop for Preventive maintenance of bottle washing machine
sop for Replacement of Battery of Electronic Devices
sop for Preparation of R.O. Antiscalant Solution
Preventive Maintenance of Rapid Cooling Steam Sterilizer
Preventive Maintenance of Rapid cooling steam Generator
sop for Preventive Maintenance of Dust Collector
sop for Replacement of HVAC filters
SOP for Battery Backup in Mobile Laminar Air Flow Trolley
sop for Replacement of Tube lights
sop for Replacement of UV light of purified water plant
sop for Cleaning of soft water storage tank and RO feed tank
sop for Preparation of Sodium hydroxide Solution
sop for Operation and regeneration of softener plant
Procedure Of Issuance Of material from Engineering Store
sop for Preventive Maintenance of Filter Press
sop for Preventive Maintenance of Sugar Transfer System
sop for Preventive Maintenance of Shrink Sleeve Machine
sop for Preventive Maintenance of ROPP Cap Elevator
sop for Preventive Maintenance of Liquid Transfer Pumps
sop for Preventive Maintenance of Liquid Tanks
Preventive Maintenance of Strip De-Foiling Machine
sop for Preventive Maintenance of Colloidal Mill
sop for Preventive Maintenance of Packing Collator Belt
sop for Checking light intensity
sop for Operation of Fire- Extinguisher
sop for Passivation of WFI Distribution Loop
sop for Analysis of Effluent Treatment Plant Water
sop for Methodology to Dispose ETP Sludge
sop for Action Plan In Case Of Electrical Power Failure
sop for Colour Coding of Piping and Accessories of Utilities
sop for Disposal of Engineering Waste
Filter Cleaning of HVAC System RLAF & LAF
Procedure for Calibration of Magnehelic Gauge
Procedure for Calibration of Vacuum Gauge
Procedure for Calibration of Pressure Gauge
sop for Procedure for Calibration of Temperature Controller
sop for Preventive Maintenance of Infrared Dryer Umbrella Type
sop for Preventive Maintenance of Battery stacker
sop for Preventive Maintenance of Lift
sop for Preventive Maintenance of Vacuum Cleaner
sop for Preventive Maintenance of laminar air flow unit
sop for Preventive Maintenance of Lozenges Manufacturing Line
sop for Preventive Maintenance of Garment Washing Machine
sop for Preventive Maintenance of Multi Column Distillation Plant
sop for Preventive Maintenance of Steam Sterilizer Autoclave
sop for Preventive Maintenance of Pure steam Generator
sop for Preventive maintenance of Depyrogenating Tunnel
sop for Preventive Maintenance of vial sealing machine
sop for preventive maintenance of Powder Filling and Rubber Stoppering machine
sop for Preventive Maintenance of Vial Washing Machine
sop for Preventive Maintenance of Inspection Table
sop for Preventive Maintenance of Hi-Cart
sop for Preventive Maintenance of Turn table
sop for Preventive Maintenance of sticker labeling machine
sop for Preventive Maintenance of Liquid Filling and Sealing Machine
sop for Preventive Maintenance of Linear Bottle Washing Machine
sop for Preventive Maintenance of De-Foiling Machine
sop for Preventive Maintenance of Strip Packing Machine
sop for Preventive Maintenance of Blister Packing Machine
sop for Preventive Maintenance of Capsule Polishing and Sorting Machine
sop for Preventive Maintenance of Capsule Filling Machine
sop for Preventive maintenance of Auto Coater
sop for Preventive Maintenance of Compression Machine
sop for preventive maintenance of packing conveyor belt
sop for Preventive Maintenance of Sifter Cum Multi mill
sop for Preventive Maintenance of Rapid Mixer Granulator
sop for Preventive Maintenance Of Fluid Bed Dryer
sop for Preventive Maintenance of Octagonal Blender
sop for Preventive Maintenance of Lifting and Positioning Device
sop for Preventive Maintenance of Paste Preparation vessel
sop for preventive maintenance of Vibro-Sifter
sop for preventive maintenance of air curtain
sop for preventive maintenance of air handling units
Preventive Maintenance of Diesel Generator
sop for Preventive Maintenance of cooling towers
sop for Preventive Maintenance of Boiler
sop for Preventive Maintenance of Air Compressor
sop for Preventive Maintenance of Chilling Plant
sop for Preventive Maintenance of Transformers
sop for Preventive maintenance of Purified Water System
sop for Break Down maintenance of Machine and Work Requisition
sop for Operation of Dust Extraction System
sop for Operation Of Effluent Treatment Plant
sop for Operation Of Diesel Generator
sop for Operation of Multi Column Distillation Plant
sop for Operation of Pure Steam Generator
sop for Operation of Air Handling Unit
sop for Operation of Cooling Tower
sop for Operation of Air Compressor
sop for Operation of Water Chilling Plant
sop for Cleaning of Pure Steam Generator
Cleaning of Over Head Water Tanks HDPE
sop for Cleaning of Water tanks
sop for Sanitization of multi column plant
sop for Sanitization of Ultrafiltration System
Sop for Chemical Sanitization of Reverse Osmosis
Sop for Sanitization of Hot Sanitization & Electro Deionization unit
sop for Sanitization of Purified Water Loop
sop for preparation of sodium meta bisulphate solution
sop for Testing of Hardness of Soft Water
I need to know about Tests of ETP in details.